Chemistry-
General
Easy
Question
Which of the following statement is correct about tropolone?

- Solubility of
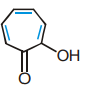 (tropolone) is less than
(tropolone) is less than 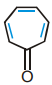 (tropone)
(tropone)
- Tropolone has more stability and aromatic character than tropone
- Tropolone has higher dipole moment than tropone
- Tropolone has lower boiling point than tropone
The correct answer is: Tropolone has more stability and aromatic character than tropone
Related Questions to study
chemistry-
In which case first has higher solubility than second?
I) Phenol, Benzene
II) Nitrobenzene, Phenol
III) o–Hydroxybenzaldehyde, p–Hydroxy benzaldehyde
IV) CH3CHO, CH3–O–CH3
V) o–Nitrophenol, p–Nitrophenol
VI) 
In which case first has higher solubility than second?
I) Phenol, Benzene
II) Nitrobenzene, Phenol
III) o–Hydroxybenzaldehyde, p–Hydroxy benzaldehyde
IV) CH3CHO, CH3–O–CH3
V) o–Nitrophenol, p–Nitrophenol
VI) 
chemistry-General
chemistry-
Arrange the following in decreasing order of their solubility in water

Arrange the following in decreasing order of their solubility in water

chemistry-General
maths-
18 points are indicated on the perimeter of a triangle ABC (see figure). How many triangle are there with vertices at these points ?
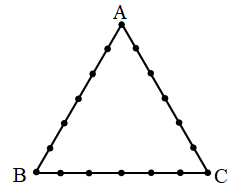
18 points are indicated on the perimeter of a triangle ABC (see figure). How many triangle are there with vertices at these points ?

maths-General
Maths-
If A and B are two events such that  and
and  then A and B are
then A and B are
If A and B are two events such that  and
and  then A and B are
then A and B are
Maths-General
maths-
Number of rectangles in the grid shown which are not squares is:

Number of rectangles in the grid shown which are not squares is:

maths-General
maths-
If  and
and  then
then 
If  and
and  then
then 
maths-General
chemistry-
The correct order of solubility in water is:
a) CH3OH
b) CH3CH2OH
c) 
d) 
The correct order of solubility in water is:
a) CH3OH
b) CH3CH2OH
c) 
d) 
chemistry-General
chemistry-
Decreasing order of melting point of compound I - IV follows:
i) 
ii) 
iii) 
iv) 
Decreasing order of melting point of compound I - IV follows:
i) 
ii) 
iii) 
iv) 
chemistry-General
chemistry-
Decreasing order of melting point of compound I to IV follows
i) 
ii) 
iii) 
iv) 
Decreasing order of melting point of compound I to IV follows
i) 
ii) 
iii) 
iv) 
chemistry-General
chemistry-
Identify the correct order of melting point of the following compounds

Identify the correct order of melting point of the following compounds

chemistry-General
chemistry-
Decreasing order of boiling point of I to IV follow
I) Methyl formate
II) Ethyl formate
III) Iso-propylformate
IV) n-propylformate
Decreasing order of boiling point of I to IV follow
I) Methyl formate
II) Ethyl formate
III) Iso-propylformate
IV) n-propylformate
chemistry-General
chemistry-
Decreasing order of boiling point of I to IV follow.
I) 
II) 
III) 
IV) 
Decreasing order of boiling point of I to IV follow.
I) 
II) 
III) 
IV) 
chemistry-General
chemistry-
The correct boiling point order is:




The correct boiling point order is:




chemistry-General
chemistry-
Arrange the following in decreasing order of their boiling points

Arrange the following in decreasing order of their boiling points

chemistry-General
chemistry-
Decreasing order of boiling point of I - IV follows

Decreasing order of boiling point of I - IV follows

chemistry-General





