Maths-
General
Easy
Question
Assertion (A) : The number of ways of arranging 6 boys and 5 girls alternately at circular table is  Reason (R) : To arrange boys and girls alternately at a circular table, they should be equal in number.
Reason (R) : To arrange boys and girls alternately at a circular table, they should be equal in number.
- A is true, R is true and R is the correct explanation for A
- A is true, R is false and R is the not the correct explanation for A
- A is true, R is false
- A is false, R is true.
The correct answer is: A is true, R is true and R is the correct explanation for A
Reason is correct explanation of the statement
Related Questions to study
chemistry-
GivenenthalpyofformationofCO2(g)andCaO(s) are–94.0KJand–152KJrespectivelyandthe enthalpyofthereaction-CaCO3(s) →CaO(s)+CO2(g) is42KJ.The enthalpyofformationofCaCO3(s)
GivenenthalpyofformationofCO2(g)andCaO(s) are–94.0KJand–152KJrespectivelyandthe enthalpyofthereaction-CaCO3(s) →CaO(s)+CO2(g) is42KJ.The enthalpyofformationofCaCO3(s)
chemistry-General
chemistry-
Change in entropy is negative for–
Change in entropy is negative for–
chemistry-General
chemistry-
The enthalpychange(DH) forthereaction,N2(g) +3H2(g) 3/4 →2NH3(g)is–92.38KJat 298K.TheinternalenergychangeDUat298Kis-
The enthalpychange(DH) forthereaction,N2(g) +3H2(g) 3/4 →2NH3(g)is–92.38KJat 298K.TheinternalenergychangeDUat298Kis-
chemistry-General
chemistry-
For a phase change H2O(l)  H2O(s)M
H2O(s)M
For a phase change H2O(l)  H2O(s)M
H2O(s)M
chemistry-General
chemistry-
The absolute enthalpy of neutral is ation of the reaction: MgO(s)+2HCl(aq)3/4→MgCl2(aq)+H2O(l) will be-
The absolute enthalpy of neutral is ation of the reaction: MgO(s)+2HCl(aq)3/4→MgCl2(aq)+H2O(l) will be-
chemistry-General
chemistry-
For which one of the following equations is D H reaction equal to DH° for the product-
For which one of the following equations is D H reaction equal to DH° for the product-
chemistry-General
Maths-
When a die is rolled twice, if the event of getting an even number is denoted by a success and the number of successes as a random variable, then distribution and mean of the variate are
When a die is rolled twice, if the event of getting an even number is denoted by a success and the number of successes as a random variable, then distribution and mean of the variate are
Maths-General
chemistry-
Enthalpy of CH4+  O2→CH3OH is negative. If enthalpy of combustion of CH4 and CH3OH are x and y respectively. Then which relation is correct-
O2→CH3OH is negative. If enthalpy of combustion of CH4 and CH3OH are x and y respectively. Then which relation is correct-
Enthalpy of CH4+  O2→CH3OH is negative. If enthalpy of combustion of CH4 and CH3OH are x and y respectively. Then which relation is correct-
O2→CH3OH is negative. If enthalpy of combustion of CH4 and CH3OH are x and y respectively. Then which relation is correct-
chemistry-General
chemistry-
Change in enthalpy for reaction 2H2O2(l) → 2H2O(l)+O2(g) If heat of formation of H2O2 (l) and H2O (l)are– 188&–286KJ/mol respectively
Change in enthalpy for reaction 2H2O2(l) → 2H2O(l)+O2(g) If heat of formation of H2O2 (l) and H2O (l)are– 188&–286KJ/mol respectively
chemistry-General
chemistry-
For there action C2H5OH(l)+3O2(g)→2CO2(g)+3H2O(l) Which one istrue -
For there action C2H5OH(l)+3O2(g)→2CO2(g)+3H2O(l) Which one istrue -
chemistry-General
chemistry-
2Zn+O2 3/2 →2ZnODG°=–616J 2Zn+S2→2ZnSDG°=–293J S2+2O2 3/4 →2SO2DG°=–408J DG°forthefollowingreactionis-2ZnS+3O2 3/4 →2ZnO+2SO
2Zn+O2 3/2 →2ZnODG°=–616J 2Zn+S2→2ZnSDG°=–293J S2+2O2 3/4 →2SO2DG°=–408J DG°forthefollowingreactionis-2ZnS+3O2 3/4 →2ZnO+2SO
chemistry-General
Maths-
A random variable has the following distribution.
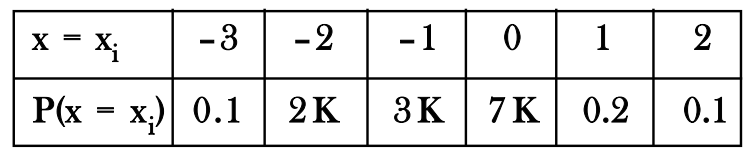
Then for the values, A = K, B = Mean, C = Variance, the ascending order is
A random variable has the following distribution.

Then for the values, A = K, B = Mean, C = Variance, the ascending order is
Maths-General
chemistry-
The heat of combustion of ethanolina bomb calorimeteris–670.48Kcalmol–1at25°C.Whatis DE at 25°C for the reaction?
The heat of combustion of ethanolina bomb calorimeteris–670.48Kcalmol–1at25°C.Whatis DE at 25°C for the reaction?
chemistry-General
Maths-
A random variable X has the following distribution
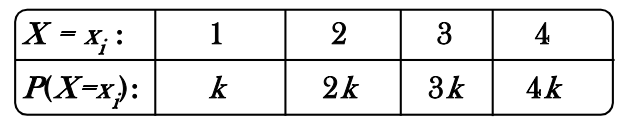
The value of  and P(X<3) are equal to
and P(X<3) are equal to
A random variable X has the following distribution

The value of  and P(X<3) are equal to
and P(X<3) are equal to
Maths-General
Maths-
If m and 2 s are the mean and variance of the random variable X, whose distribution is given by

If m and 2 s are the mean and variance of the random variable X, whose distribution is given by

Maths-General





