Base Meaning in Chemistry
Before understanding the definition of a base meaning, it is important to know the meaning of the word. The base word’s meaning is anything that supports the foundation of a thing on which it has been built. But the base meaning and definition in chemistry are different. Sometimes the base is also referred to as an alkali. The word ‘alkali’ is obtained from the Arabic word ‘al qalīy‘, which means ‘calcined ashes.’
There are four base definitions of chemistry, i.e., based on the Arrhenius concept, based on the Bronsted-Lowry concept, based on the Solvent system concept, and based on the Lewis concept.
Base Meaning Definition Based on Arrhenius’s Concept
As per Arrhenius (a Swedish chemist), a base is defined as any species that furnishes OH– ions in water. Thus, according to this concept, the substances like NaOH, KOH, Ca(OH)2, etc., are bases since they give OH- ions in water.
NaOH ⇌ Na+ + OH–
NaOH + water ⇌ Na+(aq) + OH–(aq)
Base Meaning Definition Based on Bronsted-Lowry Concept
As per Bronsted-Lowry, a base is a species (i.e., molecule or ion) that can accept a proton. In other words, a base is a species known as a proton acceptor. For instance, as Cl– ions can accept a proton (H+) to form HCl, Cl– ion acts as a base.
Cl– + H+ → HCl
Base Meaning Definition Based on Solvent System Concept
As per this concept, substances that give solvent anions when dissolved in that solvent are called bases. In other words, solvent anions can also be called base anions. For instance,
NH3 ⇌ H+ + NH2-
Here NH2- acts as a solvent anion. Hence, they behave as a base in liquid NH3.
Base Meaning Definition Based on Lewis’s Concept
Lewis defined a base as a molecule or an ion that can donate an electron pair or a lone pair of electrons to another substance. In other words, a Lewis base is an electron pair donor. It is an electron pair-rich species. Since a Lewis base loves to donate electrons to a nucleus or a positively charged ion, it is a nucleus lover and hence, is also called a nucleophilic reagent or simply nucleophile. For instance,
BF3 + OH2 → [H2O→BF3]
Here, H2O has electron pairs, and it donates them to BF3. Therefore, H2O acts as a Lewis base.
Types of Bases
Broadly, bases are classified into various categories, i.e., strong base, weak base, super base, solid base, neutral base, etc.
1. Strong bases:
A species that can remove a proton (H+) from a molecule in an acid-base reaction is termed a strong base. A strong base can dissolve completely in the aqueous solutions. It dissociates into ions in water and releases OH– ions. A strong base reacts with a strong acid and forms stable compounds (mostly salt and water).
Some examples of strong bases are
- NaOH, Sodium hydroxide
- Ba(OH)₂, Barium hydroxide
- LiOH, Lithium hydroxide
- SCa(OH)₂, Calcium hydroxide
- RbOH, Rubidium hydroxide
- KOH, Potassium hydroxide
- Sr(OH)₂, Strontium hydroxide
- Mg(OH)₂, Magnesium hydroxide
You may use many more examples of strong bases in your daily life.
2. Weak bases:
A weak base does not break out (or dissolve) completely in the aqueous solutions. In other words, the protonation process always remains incomplete on weak bases. The weak base shows incomplete ionisation in the aqueous solutions. As a result, several undissociated molecules remain in the aqueous solutions in case of a weak base.
Some examples of bases are
- Ammonium hydroxide, NH4OH
- Ammonia, NH3
- Aluminium hydroxide, Al(OH)3
- Iron hydroxide, Fe(OH)3
- Copper hydroxide, Cu(OH)2
- Lead hydroxide, Pb(OH)2
- Zinc hydroxide, Zn(OH)2
3. Superbase:
These are extremely strong bases with a high affinity for protons. Hence, deprotonation occurs more efficiently in the presence of a super base. They have very weak conjugate acids and can be produced by the reaction of alkali metals with conjugate acids.
Some examples of bases are
- Sodium hydride, NaH
- Ortho-diethynyl benzene dianion, C6H4(C2)22−
- Organolithium
- Grignard reagent
4. Neutral Base:
A neutral base incarnates a bond with a neutral acid so that the base and acid share an electron pair from the base.
5. Solid Base:
A solid base is active in solid form. These bases are operated in reactions with gaseous acids or anion exchange resins.
Some examples of bases are
- Silicon dioxide, SiO2
- NaOH mounted on alumina
Properties of Base
A base displays several characteristic properties. Some of them are
- In an aqueous or molten state, bases break into ions.
- Weak bases are poor conductors, while strong bases are good conductors of electricity.
- The pH of a base solution is always greater than 7.
- They turn the red litmus solution or paper into blue.
- They have a bitter flavour.
- They are slippery in touch and have glossy skin.
- In aqueous solutions, they release OH– ions and accept H+
- They are electron-rich species.
- They react with acids (strong and weak) to form salt and water.
- They give different colours with different pH indicators. For instance, when methyl orange is added to a basic solution, the colour changes from red to yellow.
- In the appearance of a phenolphthalein indicator, the colour of the transparent solution converts into pink if it contains a base.
- Strong and concentrated bases are corrosive. They react aggressively with organic matter and acids.
Uses of Bases in Daily Life
- Slaked lime (Ca(OH)2), or calcium hydroxide, is used to manufacture bleaching powder.
- Sodium hydroxide (NaOH) is used to manufacture paper, soap, and synthetic fiber rayon.
- Magnesium hydroxide (Mg(OH)2) is applied as an ‘antacid’ to cure indigestion and neutralise excess hydrochloric acid in the stomach.
- Calcium hydroxide (Ca(OH)2) is also used to clean the sulphur dioxide. It is caused by the exhaust found in factories and power plants.
- Sodium hydrogen carbonate (NaHCO3), or sodium bicarbonate, is used for making baking powders, as baking soda in cooking food, as an antacid to cure indigestion, and in soda acid fire extinguishers.
- Sodium carbonate (Na2CO3) is employed for softening hard water and as washing soda.
- Ammonium hydroxide (NH4OH) is used to separate grease stains from clothes.
Conclusion
You are now well-informed about the base meaning and definition chemistry from the above article. Although all these definitions are based on different concepts, all definitions agree that bases react with acids and form salt and water. In chemistry, base words mean those substances that are bitter and slippery in touch. There are many examples of bases used in general life. Bases also promote certain chemical reactions, and such reactions are termed base catalysed reactions.
Frequently Asked Questions
1. What is the difference between alkali and base in chemistry?
The difference between alkali and a base are
- All alkalis can be bases, but vice-versa is not true for all bases.
- The word alkali is used for the elements of groups I and II of the periodic table. In contrast, the base is used for the compounds containing OH–
- Alkali compounds are kinds of bases that are soluble in water, while the base neutralises the acid.
2. What are the disadvantages of the solvent system concept of acids and bases?
The main disadvantages of the solvent system concept are
- As per this concept, the acid and base definition is based on the nature of cation and anion obtained by the auto-ionisation of the solvent.
- As per this concept, the acid-base reaction only takes place in the presence of a solvent.
- This concept can’t account for the acid-base reactions occurring in non-ionising solvents like C6H6, CHCl3, etc.
3. What is the neutralisation reaction according to Lewis’s concept?
According to the Lewis concept of acids and bases, the neutralisation reaction is when a Lewis acid reacts with a Lewis base and forms a compound called an adduct or complex compound. This compound contains (Lewis base → Lewis acid) co-ordinate bond. For instance,
BF3 + NH3 → H3N→BF3
Here H3N→BF3 is an adduct.

Relevant Articles
Butanoic Acid – Structure, Properties, Uses
Butanoic Acid The carboxylic acid, butanoic acid, has the structural …
Butanoic Acid – Structure, Properties, Uses Read More »
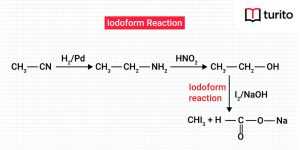
Read More >>What is Iodoform? Characteristics and Uses
Iodoform The formula for Iodoform is CHI3. It is biotic …
What is Iodoform? Characteristics and Uses Read More »
Read More >>Lattice Energy – Explanation, Factors & Formulas
Lattice Energy Lattice energy evaluates the intensity of the ionic …
Lattice Energy – Explanation, Factors & Formulas Read More »
Read More >>Lead Acetate – Definition, Properties, Uses
Lead Acetate Have you ever licked lipstick when you sketch …
Lead Acetate – Definition, Properties, Uses Read More »
Read More >>


















Comments: