In chemistry, we have heard about several elements, gases etc. But have you heard about the term dichromate? It is mainly used in the world of chemical industries. So, in this article, we will discuss dichromates in detail. We will cover all the essential information about dichromates, such as their formulas, uses, properties, etc. Let us begin.
About Dichromate
Anions of chromium and oxygen atoms are called chromate and dichromate. They are, therefore, chromium oxyanions. These terms commonly refer to materials containing these anions in a general sense. Chromate has one chromate anion, while this has two chromate anions combined.
These two anions have chemical constituents that are very similar to one another. However, they vary in appearance. Now let us look at it formula. This formula is Cr2O72–, which is a chromium oxyanion.
Typically, we refer to the compounds containing this anion as a single group using this term. It includes particles like sodium and potassium dichromate. The compounds that have dichromate as the anion also depict a vivid orange hue. 215.99 g/mol is the molar mass of this anion. Tetrahedral geometry is noticeable around the chromium atom in dichromate.
Physical Characteristics of Dichromate (Cr2O72)
- The boiling point for it is 500oC.
- The melting point for Dichromate is 398oC.
- It is odorless.
- It is a red-orange crystalline solid substance in appearance.
- It has a valency of 2.
- The pH for this is 4.
- The oxidation state is +6.
- It is moderately soluble in cold water and appreciably in hot water.
Chemical characteristics of Dichromate (Cr2O72)
- Its acidified solution produces [CrO(O2)2], which when combined with peroxide results in a deep blue colour.
4H2O2 + 2H+ + Cr2O72- + 2CrO5 + 5H2O
- It converts hydrogen sulphide into sulphur in the process of reacting with it. It also converts sulphites to sulphates, thiosulphates to sulphates, chlorides to chlorine, nitrites to nitrates, and sulphur to stannic salts.
Cr2O72- + 3H2S + 8H+ 2Cr3+ + 3S 7H2O
Uses of Dichromate
- It is used in photography to make gelatin film more durable.
- It is used in the leather industry for chrome tanning. It offers Cr(OH)3 as a moderate and is also used in dyeing.
- It calculates the volume of sulphites, iodine, and ferrous salts.
- It is used to make chrome alum, chrome yellow, and chrome red, additional chromium compounds.
Sodium Dichromate
The chemical formula for sodium dichromate, an inorganic compound with two sodium atoms, two chromium atoms, and seven oxygen atoms, is (Na2Cr2O7). Na2Cr2O7 is a crystalline solid with an orange to red appearance. The sodium dichromate compound, which contains seven oxygen atoms, is a powerful oxidising agent because it has plenty of oxygen to give to other species to oxidize them by reducing itself.
Preparation of Sodium Dichromate
Chromium(III) oxide-containing ores are used to produce sodium dichromate on a massive scale. At about 1000 °C and with air (a source of oxygen), the ore is fused with a base, usually sodium carbonate:
4Na2CrO4 + 4CO2 = 2Cr2O3 + 4Na2CO3 + 3O2
Chromium is dissolved in hot water during this phase, enabling its extraction. Other parts of the ore are now insufficiently soluble, such as the compounds of aluminium and iron. When sulfuric acid or carbon dioxide is added to the resultant aqueous extract, the dichromate results.
2Na2CrO4 + 2CO2 + H2O = Na2Cr2O7 + 2NaHCO3
Uses of Sodium Dichromate
Many things can be done with sodium dichromate, including:
- Creating chromium compounds is its main use.
- Drilling muds are made with the substance.
- Metal treatment is done with sodium dichromate.
- As a dye-making component, it is also employed.
- In the manufacturing of organic chemicals, sodium dichromate is used.
- As a corrosion inhibitor, it is typically used to stop metals from corroding.
- Many businesses that manufacture dyes use sodium dichromate.
- Plating is also done using it where a thin layer of this compound is applied to the different metallic items to prevent rusting or corrosion.
- Because of the substance’s high oxidation level, it is used in the petroleum industry.
- The production of coloured glass and ceramic glaze uses sodium dichromate as well.
Health Hazards of Sodium Dichromate
In people, potassium dichromate is a carcinogenic substance. There is proof that hexavalent chromium or chromium VI compounds cause lung cancer in both people and animals, which causes cancer. The substance can cause serious eye damage or perhaps blindness if exposed. Impaired fertility, heritable genetic harm, and harm to unborn infants are additional effects of human exposure.
Potassium dichromate can harm the gastrointestinal tract, liver, kidneys, and immune system. It primarily harms the respiratory tract, causing shortness of breath, pneumonia, ulcerations, bronchitis, and asthma. An increased risk of developing lung cancer and cancer of the sinonasal cavity is linked to this chemical, a recognized human carcinogen.
Potassium Dichromate
Potassium dichromate is a crystalline, inorganic substance that releases poisonous chromium fumes and has an orange to red tint. The potassium dichromate formula is K2Cr2O7. Potassium Dichromate is an ionic compound with two potassium ions and one negatively charged dichromate ion.
Also, two hexavalent chromium atoms are attached to three oxygen atoms and an additional oxygen atom. As a result, the final product is formed, and the potassium dichromate formula is K2Cr2O7.
In addition to being a powerful oxidizer, potassium dichromate is extremely corrosive. Although sodium dichromate mostly takes its place, this material is still employed in producing pigments, wood preservatives, and photomechanical processes.
Uses of Potassium Dichromate
Following are some of the major industrial uses of potassium dichromate:
- Potassium dichromate is used to manufacture the potassium chrome alum and in the leather tanning industry.
- Chromic acid is a substance used to clean glassware and acts as an etchant for glass products made from potassium dichromate. This can also be utilized as a raw material in the process.
- It is also employed in the building sector as a cement component, extending the time needed for the concrete mixture to set and enhancing the density and colour of the final product.
- Used as an oxidising agent and in photography screen printing in the industry.
- Organic solvents like gelatin, dye, carbon black, and gum Arabic also serve as a hardening agent. Metal printing plates used in photomechanical printing methods are created using these toughened fluids.
- A solution of potassium dichromate and dilute H2SO4 is employed when developing photographic negatives.
Ammonium Dichromate
In chemistry, Ammonium Dichromate is an inorganic compound whose formula is (NH4)2Cr2O7. Ammonium Dichromate has a +6 oxidation state, also known as hexavalent chromium. Ammonium Dichromate is also popularly known as Vesuvian Fire because it is mainly used for demonstration purposes. In the olden days, Ammonium Dichromate was used in pyrotechnics and photography.
Uses of Ammonium Dichromate
- In the initial days of photography, Ammonium Dichromate was used in pyrotechnics and lithographs. It acted as a pure nitrogen source in the labs and sometimes as a catalyst.
- In the manufacturing industry, it is mainly used as a mordant for dyeing pigments of alizarin, chrome alum, leather tanning and purifying oil.
- It is marketed as a certified insecticide, a mordant for dyeing, and a pigment.
Conclusion
Thus, we have reached the last section of today’s article, and in today’s article, we have discussed this in a detailed manner. We hope you find this article informative and that it has helped you to gain knowledge about dichromate, followed by the its formula, its properties, uses and health effects. We intend to provide detailed information about its in simple language so that you can understand them quickly.
Frequently Asked Questions on Dichromate
1. What purposes do dichromate compounds serve?
A: Compounds made of dichromate have numerous uses. They are employed as oxidising agents and in the manufacture of a variety of products, including waxes, paints, glues, etc. Because it is a hexavalent chromium product, potassium dichromate is toxic and carcinogenic.
2. Is sodium dichromate a powerful oxidizer?
A: Yes, sodium dichromate is a very powerful and effective oxidant. It is possible to oxidize primary alcohols with sodium dichromate in sulfuric acid, but this is severely restricted to the respective acid due to overoxidation via the aldehyde hydrate. Hence, it is popular as a powerful oxidizer.
3. Explain the solubility of potassium and sodium dichromate, respectively.
A: First, let us recollect the formulas of Sodium dichromate and potassium dichromate. The sodium dichromate formula is (Na2Cr2O7), and the potassium dichromate formula is (K2Cr2O7). Sodium dichromate (Na2Cr2O7) is more soluble than potassium dichromate (K2Cr2O7). This is because potassium ions are larger than sodium ions and have a lower hydration enthalpy. The covalent bonds between potassium and oxygen in potassium dichromate are also present and are difficult to break.

Relevant Articles
Butanoic Acid – Structure, Properties, Uses
Butanoic Acid The carboxylic acid, butanoic acid, has the structural …
Butanoic Acid – Structure, Properties, Uses Read More »
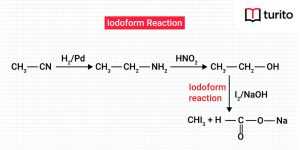
Read More >>What is Iodoform? Characteristics and Uses
Iodoform The formula for Iodoform is CHI3. It is biotic …
What is Iodoform? Characteristics and Uses Read More »
Read More >>Lattice Energy – Explanation, Factors & Formulas
Lattice Energy Lattice energy evaluates the intensity of the ionic …
Lattice Energy – Explanation, Factors & Formulas Read More »
Read More >>Lead Acetate – Definition, Properties, Uses
Lead Acetate Have you ever licked lipstick when you sketch …
Lead Acetate – Definition, Properties, Uses Read More »
Read More >>


















Comments: