Electronic Configuration
Atomic structure is a fundamental topic in the field of Chemistry. An atom contains a nucleus in its core, which is made up of protons and neutrons. In that, protons are positively charged, whereas neutrons do not possess any charges. Electrons, negatively charged particles, are present on the outer shells of the atoms surrounding the nucleus.
These electrons are arranged in a specific order inside the orbitals of the atoms. Such arrangements of electrons are termed electronic configurations.
This article will discuss how electrons are arranged and the electron configuration order in detail while having a brief look at other similar concepts.
Electron Configuration – Definition
An element’s electron configuration illustrates how electrons can be distributed in atomic orbitals. This configuration is capable of following a standard notation. Here, all the electrons that consist of atomic subshells are usually placed in a specific sequence. An atomic subshell is nothing but the notation of the number of electrons they hold.
For instance, we can say the sodium electronic configuration as: 1s2 2s2 2p6 3s1
The basic arrangement of Electronic Configuration
Configuration of electrons involves three simple terms:
- Energy level
- Orbital type
- No. of electrons present in the orbital
With that, one can simply define the electronic configuration of an atom. Look at the following examples:
1s1
Here, the 1, in front of the s, denotes the energy level.
s indicates the type of orbital.
Lastly, power 1 represents the no. of electrons in the orbital.
However, denoting the configuration based on standard notation will make the expression lengthy and complex, especially if an element contains a bigger atomic number. In those scenarios, a condensed or abbreviated notation will be highly helpful in naming the configuration rather than using the standard name.
Furthermore, in the compressed notation, we can use the square brackets form for completely filled subshells while naming the noble gases.
Hence, abbreviated sodium electron configuration is Ne 3s1 because Neon’s configuration is 1s2 2s22p6, which can be compressed to He 2s2 2p6.
Look at the following examples for further understanding:
Neon: 1s2 2s2 2p6
Aluminium: 1s2 2s22p6 3s2 3p1; it becomes Ne 3s2 3p1
Argon: 1s22s2 2p6 3s2 3p6
Calcium: 1s2 2s2 2p6 3s2 3p6 4s2; it becomes Ar 4s2
Some of the main uses of electronic configuration include:
- It is used to interpret atomic spectra
- It is useful in identifying the valency of elements
- Another major usage of electron configuration can be seen in predicting the properties of an element group. Mostly, elements that contain similar configurations are capable of exhibiting common properties.
Bohr’s atomic model is the main reason this distribution notation of electrons contained in the orbits of the atoms came into practice. It was invented by Neils Bohr and Ernest Rutherford in 1913.
How to write Electronic Configurations
Let us see how we can write electron configuration in the following passages:
Shells
The principal quantum number is the one that decides how many electrons can be accumulated inside a single shell. The principal quantum number can be denoted as n. Its formula is 2n2. Here, n indicates the number of shells.
The following tabulation will provide details about the shells, n values and total quantity of electrons that can be contained:
| Shell | Value of n | Maximum no. of electrons in the shell |
| K shell | n = 1 | 2 * 12 = 2 |
| L shell | n = 2 | 2 * 22 = 8 |
| M shell | n = 3 | 2 * 32 = 18 |
| N shell | n = 4 | 2 * 42 = 32 |
Subshells
- Electrons are distributed into the subshells based on the azimuthal quantum number. The azimuthal quantum number is represented by l.
- The azimuthal number depends on the principal quantum number value, n. Hence, when the value of n is 4, it means four various subshells are possible.
- When n = 4, the subshells corresponds to l = 0, l = 1, l = 2 and l = 3. They are termed the s, p, d and f subshells respectively.
- The highest quantity of electrons a subshell can store is provided by the formula: 2 * (2l + 1).
- Hence, one can understand that the s, p, d and f subshells can contain a maximum of up to 2, 6, 10 and 14 electrons, respectively.
The following tabulation gives details about all the possible subshells for n values up to 4:
| Value of Principal Quantum Number | Value of Azimuthal Quantum Number | Resulting Subshell in the Electron Configuration |
| n = 1 | l = 0 | 1s |
| n = 2 | l = 0 | 2s |
| l = 1 | 2p | |
| n = 3 | l = 0 | 3s |
| l = 1 | 3p | |
| l = 2 | 3d | |
| n = 4 | l = 0 | 4s |
| l = 1 | 4p | |
| l = 2 | 4d | |
| l = 3 | 4f |
Therefore, we can conclude that the 1p, 2d and 3f orbitals do not exist. It is because the principal quantum number’s value is always higher than the azimuthal quantum number’s value.
Notation
- We can write the electronic configuration of an atom with the assistance of subshell labels.
- All these labels have the shell number provided by the principal quantum number. Along with that, a subshell name will be provided by the azimuthal quantum number. And finally, they contain the total quantity of electrons inside the subshell.
- For instance, in case 2 electrons are filled in the first shell’s s subshell, the outcome would be 1s2.
- With the assistance of these subshell labels, we can write the magnesium’s electronic configuration as 1s2 2s2 2p6 3s2.
Filling the Atomic Orbitals
Under this heading, we will be discussing various rules and principles that explain how to fill the atomic orbitals using electrons. Some of the most common ones are:
1. Pauli Exclusion Principle
- This principle explains that only two electrons can fit in an orbital. These electrons should have opposite spins.
- Pauli Exclusion Principle states that no two electrons in a single atom contain the same values for all four quantum numbers.
- Hence, in case the azimuthal, principal and magnetic numbers are the same of both the electrons, they will perform opposite spins.
2. Aufbau Principle
- Aufbau is a German word meaning ‘build up’.
- In the Aufbau principle, it has been described that the electrons will occupy the orbitals containing lower energies before going on to fill the higher ones.
- We can calculate the energy of the orbital by summing the principal quantum number and azimuthal quantum number.
- The principal proves that the electrons are filled in the below mentioned order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p…
The following is the illustration of the same:

Apart from that, there are many exceptions to this principle, namely copper and chromium. We can explain these exceptions based on the stability given by partially filled or fully filled subshell.
3. Hand’s Rule
- Hand’s rule illustrates the order where electrons are filled. The electrons have to fill all the orbitals that belong to the subshell.
- It indicates that all the orbitals contained in a provided subshell are solely occupied by electrons until there is a presence of a second electron that comes to accompany it.
- To maximise the total spinning ratio, the single electron orbitals can perform the same spin.
Following is the illustration of the same:

How to represent the Electronic Configuration of the atom?
Below mentioned are some of the elements of electronic configuration:
1. Hydrogen’s Electron Configuration
Hydrogen’s atomic number is 1. Hence, a hydrogen atom consists of 1 electron. It is placed in the first orbit’s s subshell. Hydrogen’s electronic configuration is 1s1. Following is the illustration of the same:

2. Oxygen’s Electronic Configuration
Oxygen’s atomic number is 8. It means that an oxygen atom consists of 8 electrons. Following is the order where electrons are filled inside an oxygen atom:
K shell: 2 electrons
L shell: 6 electrons
Hence, oxygen’s electron configuration is 1s2 2s2 2p4. Below given is the illustration of the same:
3. Chlorine’s Electronic Configuration
The atomic number of chlorine is 17. It indicates that it contains 17 electrons. They are distributed in the given manner:
K shell: 2 electrons
L shell: 8 electrons
M shell: 7 electrons
Therefore, chlorine’s electron configuration is 1S2 2s2 2p6 3s2 3p5
Following is the illustration of the same:

Conclusion
Electron configuration is an essential topic in order to understand the orbital arrangement of electrons inside each atom. Hence, this educational blog has a detailed introduction to the electronic configuration and electron configuration order.
In addition, we have also gone through some of the principles where we have seen how electrons are filled based on certain rules and regulations.
Frequently Asked Questions
1. Mention the electronic configuration of noble gases.
The following list provides details regarding the electronic configurations of noble gases:
-
- Helium He:1s2
- Neon Ne:He 2s2 2p6
- Argon Ar:Ne 3s23p6
- Krypton Kr:Ar 3d10 4s2 4p6
- Xenon Xe:Kr 4d10 5s2 5p6
- Radon Rn:Xe4f14 5d10 6s6 6p6
2. Describe the copper’s electron configuration
Copper’s electronic configuration is Ar 3d10 4s1. It does not agree with the Aufbau principle because it only has a smaller energy gap between the 3d and 4s orbitals. Therefore, the fully-filled d-orbital can offer more stability compared to the partially filled one.
3. What is the importance of electron configuration?
The major purpose of electron configuration is to give insight into the elements’ chemical behaviour by assisting them in demonstrating the valence electrons of an atom. In addition, it helps in classifying the elements into various blocks, namely s, p, d and f block elements. Doing so will make it easier to study the atoms’ properties.

Relevant Articles
Butanoic Acid – Structure, Properties, Uses
Butanoic Acid The carboxylic acid, butanoic acid, has the structural …
Butanoic Acid – Structure, Properties, Uses Read More »
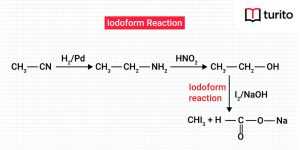
Read More >>What is Iodoform? Characteristics and Uses
Iodoform The formula for Iodoform is CHI3. It is biotic …
What is Iodoform? Characteristics and Uses Read More »
Read More >>Lattice Energy – Explanation, Factors & Formulas
Lattice Energy Lattice energy evaluates the intensity of the ionic …
Lattice Energy – Explanation, Factors & Formulas Read More »
Read More >>Lead Acetate – Definition, Properties, Uses
Lead Acetate Have you ever licked lipstick when you sketch …
Lead Acetate – Definition, Properties, Uses Read More »
Read More >>


















Comments: