Hydrochloric acid is an inorganic compound containing hydrogen and chlorine. It is a corrosive acid. Hydrochloric acid is also called hydrogen chloride or muriatic acid. Both hydrochloric acid and hydrogen chloride share the same molecular formula, HCl, as they are the same compound. However, they differ in the states. Hydrogen chloride occurs in the form of a gas at room temperature and pressure.
When hydrogen chloride gas gets dissolved in water, it gives HCl acid. So, to differentiate the two, a subscript depicting the state of matter is written with the chemical formula — HCl(g) and HCl(aq). The following article explores HCl chemistry in detail.
Hydrochloric Acid Structure – HCL
Hydrogen chloride or hydrochloric acid is a simple diatomic molecule wherein the two atoms– hydrogen and chlorine, are held together by a single covalent bond. The bond is polar because the chlorine atom is more electronegative than the hydrogen atom. While the electronegativity of chlorine is 3.16, hydrogen has an electronegativity of 2.2.

Lewis Structure of hydrochloric Acid
The compound contains
- One hydrogen and one chlorine atom. It is diatomic.
- It has one single bond or a pair of bonding electrons.
- It has three pairs of nonbonding electrons.
Hydrogen chloride readily dissolves in water because:
- Both hydrogen and water are polar in nature due to the difference in the electronegativity of their atoms.
- HCl has the ability to dissociate into ions. So, the water molecule pulls the hydrogen ions from the chlorine ions.
Preparation of Hydrochloric acid – HCl
The following method for hydrochloric acid preparation is popular in laboratories as well as for commercial-scale hydrogen chloride preparation. In this method, sodium chloride is nested with hot and concentrated sulphuric acid.
NaCl + H2SO4 → NaHSO4 + HCl
NaHSO4 + NaCl → Na2SO4 + HCl
Properties of Hydrochloric Acid
Hydrochloric acid is a strongly acidic, colourless, and viscous liquid with a distinctively pungent smell. Its distinct smell makes it easy to recognise in laboratories. HCl is used in leather processing and gelatin production. Most of the physical properties of HCl, like density, pH, melting point, and boiling point, depend on its molar concentration.
Some important properties of HCl are stated below:
- Molecular Weight/ Molar Mass: 36.458 g/mol
- Odour: Pungent smell
- Appearance: Transparent liquid
- Boiling Point: Concentration-dependent
- Melting Point: Concentration-dependent
Chemical Properties of Hydrochloric Acid
Gaseous hydrogen chloride readily forms chlorides with active metals, their carbonates, oxides, and hydroxides. The reactions mostly take place in the presence of humidity.
It reacts with metals and displaces hydrogen gas. On reacting with simple (metal) oxides and hydroxides, it forms metal chloride and water– the common neutralisation reaction.
HCl Oxidation
HCl reacts with potassium permanganate or potassium dichromate to liberate chlorine gas.
2KMnO4 + 16 HCl → 2 KCl + 2 MnCl2 + 5 Cl2 + 8 H2O
2K2Cr2O7 + 14 HCl → 2 KCl + 2 CrCl3 + 3 Cl2 + 7 H2O
Reaction With Carbonates
Hydrochloric acid reacts with carbonates to produce carbon dioxide.
Na2CO3 + 2 HCl → 2 NaCl + H2O + CO2
Reaction With Bicarbonates
Hydrochloric acid reacts with hydrogen carbonates to give carbon dioxide.
NaHCO3 + HCl → NaCl + H2O + CO2
Reaction with Sulphites
Hydrochloric acid reacts with sulphites to give sulphur dioxide gas.
Na2SO3 + 2 HCl → 2 NaCl + H2O + SO2
Aqua Regia Formation
When concentrated hydrochloric acid and concentrated HNO3 combine in a 3:1 ratio, aqua regia is formed. This mixture can dissolve metals such as platinum and gold to form their respective chlorides.
Dissociation of HCl
HCl is a strong acid. But what do you mean by a strong acid?
According to Arrhenius, an acid is a substance that forms hydrogen ions or protons upon dissociation.
The ease and quantity of hydrogen ion liberation make the acid strong or weak. HCl is categorised as a very strong acid because it dissociates in water. Thus, as a proton donor, HCl satisfies all the characteristics of an acid. It serves as a base only when it is a proton recipient, and that happens when it reacts with a higher acid dissociation constant substance or a superacid. The aqueous form of hydrogen chloride is also acidic. The dissociation reaction is shown in the following equation:
HCl + H2O → H3O+ + Cl−
Hydrochloric acid Uses – HCL
Earlier, it was dissolved in liquids and dumped in oceans as its industrial uses weren’t released. Later, with the advancement of scientific study, major uses of industrial uses came forward, including
Production of Organic Compounds
It is used in the production of organic compounds such as dichloromethane or vinyl chloride, plastics, bisphenol A, and more.
Production of Inorganic Compounds
It is useful in preparing compounds used for water treatment. Aluminium and hydrochloric acid find usage in the production of poly aluminium chloride (PAC), aluminium carbohydrate, and iron(III) chloride for water treatment.
It is also used in the regeneration of ion exchange resins. It is also used to rinse the resin cations.
For Table Salt Purification
HCl is popular for purifying table salts. Further, it is also used for regulating the acidity (pH) of solutions. HCl is efficient in controlling the pH of foods, pharmaceutical products, and water.
Removing Metal Stains
It serves as a strong chemical to remove rust or stains from iron, copper, and other metals. However, its diluted form is used for cleaning purposes, such as tiles in bathrooms and kitchens.
It is also used as a disinfectant or in the textile industry for bleaching clothes, leather processing, and more.
For Oil Production
HCl is used in oil production. It is a rock that leads to large-pore structure formation due to the reactions that help in oil production.
Other Uses
- Making glue and gelatin
- Producing glucose and corn sugar from starch
- Manufacturing plastics and synthetic rubber
- Refining cane sugar
- Manufacturing aqua regia for dissolving gold and platinum
- It is used in the process of chloride production.
- For fertiliser production.
- In textile industries and the manufacturing of dye
- For refining of metals
- To regulate the PH of solutions
Is Hydrochloric Acid Found in the Human Body?
Abdominal parietal cells give rise to HCl in the body. The process involves ATP energy for exchanging potassium ions present in the stomach with the parietal cell hydrogen ions. Consequently, both hydrogen and chloride ions occur in the stomach lumen.
The secretion of the stomach comprises various enzymes and hydrochloric acid. The mucus coating protects the stomach lining from the harmful effects of HCl.
The HCl in the stomach participates in the breakdown of foods and digests them. The gastric juice contains hydrochloric acid that splits up the proteins. It also kills bacteria in the stomach and eliminates viruses, thus protecting the human body from infections.
The concentration of hydrochloric acid in the stomach is 0.5 per cent. The balanced stomach pH is about 1.0-2.0. The low pH of the stomach makes it free from microbes.
Hydrochloric Acid on Skin
It is important to understand that the hydrochloric acid present in the stomach is very mild in concentration and the hydrochloric acid in laboratories is quite strong. So, one has to be very careful while using hydrochloric acid.
If low concentrations of HCl gas or hydrochloric acid come into contact with skin, it causes erythema and inflammation. However, high concentrations can lead to chemical burns on the skin and even mucous membranes when inhaled.
If you happen to drop concentrated hydrochloric acid on your skin, you must immediately take the following measures:
- Rinse the affected area thoroughly with clean water as soon as possible.
- You must ensure that the water runs off the affected area and does not pool on the skin.
- Also, you must not try to rub or wipe the area.
Hydrochloric Acid Safety
If you have swallowed hydrochloric acid, seek medical attention right away and do not induce vomiting unless instructed to do so or unless you are more than 15 minutes away from the hospital (only induce vomiting on a person who is conscious).
In the event of skin contact, remove all contaminated clothing, footwear, and accessories and immediately wash the affected area with plenty of soap and water. Clothing that has been contaminated must be washed before it can be worn again. If your symptoms persist, see a doctor.
Conclusion
Hydrochloric acid is the aqueous solution of hydrogen chloride. It is a corrosive yet useful chemical that finds applications in various industries. HCl is naturally present in our body in the form of gastric HCl, which has a 0.5% concentration.
Frequently Asked Questions
1. What is the most popular use of hydrochloric acid?
A. Hydrochloric acid is a potent chemical reagent for manufacturing polyvinyl chloride for plastics. It is frequently used for cleaning and disinfecting households. Diluted HCl helps in descaling areas. It is used in gelatin processing and also as a food additive in the food industry.
2. How would you neutralise hydrochloric acid?
A. Strong bases react with hydrochloric acid to give a neutralisation reaction that produces salt and water. You can use sodium bicarbonate or baking soda in the area of the hydrochloric acid spill to neutralise the acid. Apply sufficient baking soda before it begins to fizz. The neutralised hydrochloric acid can then be flushed using excessive quantities of water.
3. Is hydrochloric acid weak or strong?
A. Heavy acids are those that dissociate completely into their ions. Contrastingly, weak acids dissociate partially. Sulphuric acid, hydrochloric acid, and nitric acid are some of the most popular strong acids.
4. What is Hydrochloride?
A. Hydrochloride is the product obtained when hydrochloric acid reacts with an organic base. An organic base is a hydrocarbon that serves as a base or proton recipient. Some examples of organic bases are as follows:
- Pyridine C5H5N
- Methylamine CH3NH2
- Benzimidazole C7H6N2
5. Is Hydrochloric Acid Harmful to Humans?
Chemical burns from hydrogen chloride can be very severe if they come into contact with skin or other tissues. Blindness can result from exposure to stomach acid in the eyes. The acid’s concentration and how long it remains in contact with the tissues determine how severe the burns will be.

Relevant Articles
Butanoic Acid – Structure, Properties, Uses
Butanoic Acid The carboxylic acid, butanoic acid, has the structural …
Butanoic Acid – Structure, Properties, Uses Read More »
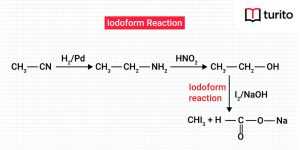
Read More >>What is Iodoform? Characteristics and Uses
Iodoform The formula for Iodoform is CHI3. It is biotic …
What is Iodoform? Characteristics and Uses Read More »
Read More >>Lattice Energy – Explanation, Factors & Formulas
Lattice Energy Lattice energy evaluates the intensity of the ionic …
Lattice Energy – Explanation, Factors & Formulas Read More »
Read More >>Lead Acetate – Definition, Properties, Uses
Lead Acetate Have you ever licked lipstick when you sketch …
Lead Acetate – Definition, Properties, Uses Read More »
Read More >>


















Comments: