Lithium Hydroxide
Lithium hydroxide is a synthetic chemical compound having the chemical formula LiOH. It is a white, hygroscopic, crystalline compound that is somewhat soluble in ethanol and water. It is created in clinical laboratories by reacting water with lithium oxide or lithium. Amongst alkali metal hydroxides, lithium oxide is the base with the lowest stability. Its pH is somewhere between 10 and 11. It is the very first alkali compound in the periodic table.
Lithium is derived from the word “Lithos”. It is a word used for stone in Greek. Lithium (Li), an alkali metal, is a chemical element from Group 1 of the periodic table. Lithium is a metal primarily used in vegetables and grains for dietary purposes. It can be used in multiple forms in a variety of supplements. Lithium is termed the lightest metal in solid elements. Having a 1,342°C boiling point and an oxidation state of +1. It is a member of the s-block.
Rechargeable batteries for electric vehicles, mobiles, electronic devices, laptops, and digital cameras all use lithium. Hydrogen oxide, hydroxide, a chemical compound comprising one or more groups. It comprises an oxygen atom and a hydrogen atom joined by a covalent bond. Where a hydrogen atom carries a negative electric charge, it’s a component in food preservatives.
Lithium Hydroxide: As a Compound
Below is a list of key characteristics.
| Compound | Values |
| IUPAC Name | Lithium Hydroxide |
| Lithium Hydroxide formula | LiOH |
| Lithium Hydroxide molar mass | 24 g/mol |
| Segmentation Temperature | 924°C |
| Vapour Pressure | Negligible at 20°C |
| Odour | Pungent odour |
| Solubility | 12.8g/100ml in water at 20°C |
| Density | 2.54 g/cm3 |
| Flame Resistance | Non-Flammable |
| Components | Both dry powder, Liquid |
| Colour | Colourless to white |
Lithium Hydroxide Formula
The chemical formula for Lithium Hydroxide can be written as LiOH. The molar mass of lithium hydroxide is 23.91 g/mol. There are two types of LiOH: anhydrate and monohydrate. The metastable monomeric compound is linear and moderately flexible. In the solid state, hydrated LiOH is anhydrous and exists as a monohydrate. It seems to be the only alkali hydroxide that lacks polymorphism.
Lithium Hydroxide: Structure
Lithium hydroxide’s structure (or framework) consists of an oxygen atom, a hydrogen atom, and a lithium-ion. It has a high degree of reactivity and can combine with several other compounds to create new compounds. It crystallizes in a tetragonal structure with a P4/mm configuration with two formulas per unit cell at ambient temperature and pressure.
Lithium atoms are arranged in layers in square lattices, each square topped by an alternating layer of a hydroxide ion above and beneath the lithium layer. A deformed tetrahedral environment of oxygen atoms surrounds each lithium atom, and each oxygen ion is fourfold correlated with lithium atoms.
Informative details
|
Lithium Hydroxide: Preparation
Lithium hydroxide is prepared when Lithium Carbonate chemically reacts with Calcium Hydroxide.
Li2CO3 + Ca(OH)2→ 2LiOH + CaCO3
In this process or reaction, Calcium Carbonate is removed from the solution, evaporating and crystallizing. LiOH•, H2O a monohydrate, is the resultant product. The hydrate is heated beyond 100°C in a vacuum or without carbon dioxide to produce the anhydrous chemical. In addition, the reaction of lithium oxide and water creates hydroxide.
Lithium Hydroxide: Physical Properties
- Lithium hydroxide is an odourless natural substance.
- It improves the dye’s quality.
- It has a hygroscopic, solid white look.
- Lithium Hydroxide has a density of 1.46 g/cm3.
- LiOH has melting and boiling temperatures of 462 °C and 924 °C, respectively.
- It dissolves nicely in water but not well in alcohol.
Lithium Hydroxide: Chemical Properties
- In an industrial setting, lithium hydroxide is produced by combining lime with lithium ore or a salt manufactured from the ore.
- It resembles the group 2 hydroxides more so than the group 1 hydroxide.
- It’s basic. But compared to sodium or potassium hydroxide, it is less basic.
- It goes through processes of neutralization with acids, such as hydrochloric acid:
LiOH + HCl → LiCl + H2O
- Lithium Oxide is produced when lithium hydroxide is heated over 800 °C in a vacuum.
2LiOH → Li2O + H2O
- It produces lithium carbonate and readily absorbs carbon dioxide.
2LiOH + CO2→ Li2CO3 + H2O
- Lithium Hypochlorite is produced when chlorine is added to lithium hydroxide.
LiOH + Cl2→ LiOCl + HCl
- The saponification procedure involves the use of lithium hydroxide.
LiOH + CH3(CH2)16COOH→ CH3(CH2)16COOLi + H2O
Lithium Hydroxide: Nuclear properties
Lithium contains two isotopes of mass number 6 (92.5 per cent) and 7 but does not naturally emit radiation (7.5 per cent). Lithium-7 to Lithium-6 ratio ranges from 12 to 13.
In their groundbreaking research, Irish physicist Ernest Walton and British physicist John Cockcroft utilized lithium as the target metal in 1932. Each lithium nucleus that absorbed a proton transformed into two helium nuclei. Slow neutron bombardment of lithium-6 results in the synthesis of helium and tritium (3H); this reaction significantly contributes to tritium production. The tritium so created is used in producing hydrogen bombs and other applications, such as producing a radioactive hydrogen isotope for scientific research and study.
Uses of Lithium Hydroxide
- It is often used to prevent corrosion.
- Submarines employ LiOH as a carbon dioxide scrubber.
- The ceramic and paint industries both employ lithium hydroxide.
- Since it is mostly water soluble, it is frequently used to create lithium soaps, which are then used to create multifunctional greases.
- Lithium carbonate and lithium hydroxide are both combined and used as a dye. The reaction of both increases the dye’s quality.
- Additionally, it is employed in the production of several lithium salts.
- It is incorporated as a supplement in the electrolyte of alkaline storage batteries.
- It serves as an effective and portable carbon dioxide absorber for environmental purposes.
- It is used in homes to create cleaning solutions, laundry detergent, and fabric conditioners.
- Today’s leading battery manufacturers employ it because its cathodes have advantages over other compounds in terms of better power density, larger power capacity, extended life cycles, and improved safety features.
- It is produced at a lower cost than brine and is frequently used in the production of ceramics, plastics, metallurgical powders, and rechargeable batteries.
- It is also frequently used to produce lithium carbonate and water in respiratory gas purification devices for submarines, spacecraft, and rebreathers to eliminate carbon dioxide from breathing gas.
| Note: Exposure to lithium oxide can cause significant irritation in the eyes, skin, and mucous membranes |
Conclusion
- Lithium hydroxide is an inorganic substance having the chemical formula of LiOH. It is a white, hygroscopic, crystalline substance that is somewhat soluble in ethanol and soluble in water.
- It is created in laboratories by reacting water with lithium or lithium oxide.
- Lithium oxide is the base with the lowest stability in all alkali metal hydroxides. Its pH is somewhere between 10 and 11. It is the very first alkali in the periodic table.
- Due to its excellent water resistance and adaptability in low and high temperatures, lithium stearate is used most frequently in lubricants.
- Today’s leading battery manufacturers employ it because its cathodes have advantages over other synthetic chemicals in terms of better power density, larger power capacity, prolonged life cycles, and improved safety features.
- In an industrial setting, lithium hydroxide is created by combining lime with lithium ore or a salt manufactured from the ore.
Frequently Asked Questions
Q1.Is lithium hydroxide acidic or basic?
A:It is basic. Since it releases two positive Li-ions and a negative OH ion when it dissolves in a solution, it can totally dissolve in water since it is a strong base.
LiOH + (aq) → Li+(aq) + OH-(aq)
Q2.What are lithium hydroxide’s pH and basicity values?
A:Ans. Lithium hydroxide’s pH value is greater than 7. Because every molecule with a pH value of more than 7 is considered basic as it produces OH- after dissolving in water. Its basicity, though, is -0.04.
Q3.What kind of reaction do water and lithium have?
A:When lithium reacts with water, it results in an exothermic reaction. Where both lithium and water produce hydrogen gas and lithium hydroxide as their end products.
2Li (solid) + 2H2O (liquid) → 2LiOH (aq) + H2 (gas)
As a soluble salt, lithium hydroxide separates into lithium cations and hydroxide anions in an aqueous solution.
Q4.What happens when ethanol and lithium hydroxide interact?
A:The following reaction might occur when ethanoic acid and lithium hydroxide interact. The following is the balanced chemical equation:
LiOH (aq) + CH3COOH(aq) → LiCH3COO(aq) + H2O (l)

Relevant Articles
Butanoic Acid – Structure, Properties, Uses
Butanoic Acid The carboxylic acid, butanoic acid, has the structural …
Butanoic Acid – Structure, Properties, Uses Read More »
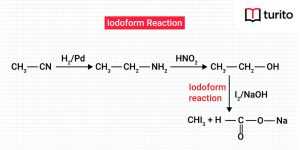
Read More >>What is Iodoform? Characteristics and Uses
Iodoform The formula for Iodoform is CHI3. It is biotic …
What is Iodoform? Characteristics and Uses Read More »
Read More >>Lattice Energy – Explanation, Factors & Formulas
Lattice Energy Lattice energy evaluates the intensity of the ionic …
Lattice Energy – Explanation, Factors & Formulas Read More »
Read More >>Lead Acetate – Definition, Properties, Uses
Lead Acetate Have you ever licked lipstick when you sketch …
Lead Acetate – Definition, Properties, Uses Read More »
Read More >>


















Comments: