You must be aware that the periodic table has 18 groups. Each group has its own different properties and characteristics. The same goes for the halogen group. The elements in the halogen family have a small size and the highest electronegativity among other groups.
Due to their compact size and high electronegativity, they form oxoacids. Now the question is, what are oxoacids? In oxoacids of halogens, a halogen atom (other than fluorine) forms one or more bonds with an oxygen atom or hydroxide atoms. In this way, all the halogens form oxoacids. But each has different properties, uses, structures, and many other features.
In this article, you will learn about perchloric acid or oxyacid chlorine.
Structure of Perchloric Acid, HClO4
As it is an oxoacid of chlorine, the central atom of perchloric acid or hydrochloric acid is the chlorine atom. Four oxygen atoms covalently bond it. To be precise, the chlorine atom in it is surrounded by three oxygen atoms and one hydroxide group.
All three oxygen atoms form double bonds with the chlorine atom. It is to fulfill the octet rule. As a chlorine atom can extend its valence shell, it forms double bonds with three oxygen atoms. The hydroxide group is attached to the chlorine atom through a single bond with it. The hydrogen in the hydroxide group makes perchloric acid the strongest acid, as it releases easily in aqueous solutions.
The structure of this is given below.

Preparation of Perchloric Acid, HClO4
There are a few ways to prepare this acid. Some of them are
1. From Perchlorate Acid, HClO3:
It is prepared by heating perchlorate acid.
3HClO3 + heat → HClO4 + Cl2 + 2O2 + H2O
2. From Potassium Perchlorate, KClO4:
Anhydrous perchloric acid is obtained by distilling a mixture of KClO4 with concentrated H2SO4 under reduced pressure.
KClO4 + H2SO4 → HClO4 + KHSO4
3. From Barium Perchlorate, Ba(ClO4)2:
This solution is obtained by treating Ba(ClO4)2 with a calculated quantity of dilute H2SO4 and then removing the insoluble BaSO4 by filtration.
Ba(ClO4)2 + H2SO4 → 2HClO4 + BaSO4↓
4. From Ammonium Perchlorate, NH4ClO4:
The perchloric acid or perchlorate acid solution can also be obtained by adding NH4ClO4 dissolved in concentrated HCl to warm concentrated HNO3 and then evaporating.
NH4ClO4 + 8HCl + 3HNO3 → HClO4 + 2N2O + 4Cl2 + 7H2O
Properties of Perchloric Acid, HClO4
The properties of perchloric acid or hyperchloric acid are categorized into two classes, i.e., physical and chemical.
The Physical Properties of Perchloric Acid are:
- Perchloric acid, HClO4, is commonly known as hydroxidotrioxidochlorine or hyperchloric acid.
- It has a colorless, clear, and odorless physical appearance.
- Generally, it exists as an aqueous solution.
- It is corrosive to metals and tissues.
- The chemical formula of perchloric acid is HClO4.
- The melting point of HClO4 is −17°C and its boiling point is 203°C.
- The molecular weight of perchloric acid, HClO4, is 100.46 g/mol.
- Its density is 1.768 g/cm3.
- The pKa value of its aqueous solution is −15.2±2.0.
The Chemical Properties of Perchloric Acid, HClO4, are
- HClO4 is the strongest acid of all the acids.
- It is a highly dangerous acid and produces severe wounds on the skin.
- It is a powerful oxidizing agent and inflames paper and wood.
- It forms hydrates with 1, 2, 2.5, 3, and 3.5 molecules of water up to crystallization.
- HClO4 is not reduced by nascent hydrogen but gets reduced to chloride by strong reducing agents such as SnCl2, CrCl2, etc.
- This acid is unstable and decomposes with the explosion on heating. Sometimes, merely stood for a few days, even in the dark.
- Perchloric acid solution is quite stable and does not decompose.
- On dehydration with P2O5 at -10℃, it gives Cl2O7, the anhydride of perchloric acid.
- 2HClO4 + P2O5 → Cl2O7 + 2HPO3
This reaction has been used for the preparation of Cl2O7.
- Anhydrous perchloric acid, HClO4, is a colorless mobile hygroscopic, and oily liquid.
- Anhydrous perchloric acid fumes strongly in moist air.
- Anhydrous HClO4 dissolves in water with a hissing sound due to the liberation of removing much heat.
- The metals like Zn, Fe, etc., dissolve in the aqueous solution of the acid and form soluble perchlorates.
- Zn + 2HClO4(aq) → Zn(ClO4)2(aq) + H2↓
- When a suspension of iodine is heated with HClO4, paraperiodic acid (H5IO6) is obtained.
- 2HClO4 + I2 + 4H2O → 2H5IO6 + Cl2
Uses of Perchloric acid, HClO4
This is used for many purposes. Some of the major uses of perchloric acid are given below:
- It is used as an oxidizer to separate potassium and sodium in many laboratory tests.
- It is a precursor to ammonium perchlorate, a vital rocket fuel component.
- It is used in making explosives.
- It is used as a critical compound in the space industry.
- Decomposition of perchloric acid on heating produces toxic and corrosive fumes.
- It is used in the plating of metals.
- It is used in systems etching Liquid Crystal Display (LCD).
- It is for electropolishing or etching of chrome and molybdenum.
- It functions as a reagent for determining the 1H-Benzotriazole.
- It plays a major role in material extraction from their ores.
- Because of its super acidic property, this is one of the major components of Bronsted-Lowry.
- Because of its unique property, it is used in analytical chemistry.
- It is also used in the electronics industry.
- It is a strong oxidant, making it a fire and explosion-hazardous substance.
Health Hazards Caused by Perchloric Acid
Although this is very useful for many purposes, it causes major health issues.
- Inhalation of vapors of this compound causes a burning sensation in the nose, throat, and lungs.
- Prolonged exposure to this compound causes vomiting.
- Ingestion of this compound can cause blistering and burns in the stomach.
- It is highly corrosive to human skin as well.
- Because of its oxidizing nature, it is highly reactive toward most metals. It liberates irritating, corrosive, and toxic gases when heated. Thus, this compound must be handled with adequate safety measures.
Some Points to Remember
The following are the important points:
- It is a chlorine oxoacid with the formula HClO4
- It is also called Hyperchloric acid or hydroxidotrioxidochlorine
- It is an odorless and colorless aqueous solution at a standard temperature.
- It is dangerously corrosive and thus readily forms explosive mixtures.
- It acts as a strong oxidizer when hot but only shows acidic properties at room temperature.
- Though it has vast uses, it has a key usage in the space industry for producing ammonium perchlorate. This ammonium perchlorate is a major component of rocket fuel.
- Commercially, this acid is available in water with a concentration of 70% (by weight).
- There are two major ways of industrial production.
- It is one of the strongest Bronsted–Lowry acids.
Safety Measures
One must follow the given safety precautions while working with perchloric acid in the laboratory.
- Personal laboratory trainers only handle it.
- Use appropriate protective measures while working with it.
- Avoid using it with organic substances like cellulose, lipids, fats, etc.
- Never heat perchloric acid in an oil bath or on an open flame.
- Please do not use it with other chemicals before knowing its nature with them.
- Wash down perchloric acid hoods after each use.
- Do not distill perchloric acid in a vacuum because an unstable anhydride will form.
Conclusion
HClO4, Perchloric acid or chlorine oxoacid, is produced by the reaction of alkali perchlorates with hydrochloric acid or barium perchlorate with sulphuric acid in a laboratory. Although it is used to separate potassium and sodium, its chief function is to prepare a vital rocket fuel component, i.e., ammonium perchlorate. Also, it has an advantage in many laboratory tests and industrial processes.
Frequently Asked Questions
1. Why Should Perchloric Acid be Used With Utmost Care and Precaution?
A. Perchloric acid or perchlorate acid is one of the powerful mineral acids. It has a high oxidizing power at high temperatures. As a result, it is very suitable for the digestion of organic matter such as proteins, fats, and lipids. The perchlorates produced during its decomposition are easily soluble in water. One must handle it with extreme care due to its rapid reactivity with organic matter because sometimes it can become explosive.
2. Is Perchloric Acid the Strongest Acid?
A. Yes. It is one of the more vigorous acids. It is because of the presence of perchlorate acid or perchlorate. This perchlorate is a weak nucleophile and does not show hydrolysis compared to other acids. As a result, it releases H+ ions easily in the aqueous state, forming a strong acid.
3. What Happens When Perchloric Acid Reacts With Metals?
A. It reacts with metals and forms flammable or explosive gas. It is highly oxidant and can cause a fire or explosive hazard when it comes in contact with organic matter. When it gets heat, it decomposes and produces toxic and corrosive fumes.
4. What Other than Perchloric Acid, and Oxy-acids does Chlorine Atom Form?
A. Chlorine forms four oxy-acids, which are hypochlorous acid (HCl+1O), chlorous acid (HCl+3O2), chloric acid (HCl+5O3), and perchloric acid (HCl+7O4). The oxidation states of chlorine atoms are +1, +3, +5, and +7, respectively, in these acids.

Relevant Articles
Butanoic Acid – Structure, Properties, Uses
Butanoic Acid The carboxylic acid, butanoic acid, has the structural …
Butanoic Acid – Structure, Properties, Uses Read More »
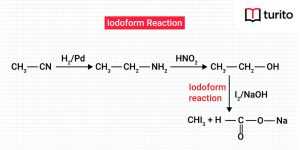
Read More >>What is Iodoform? Characteristics and Uses
Iodoform The formula for Iodoform is CHI3. It is biotic …
What is Iodoform? Characteristics and Uses Read More »
Read More >>Lattice Energy – Explanation, Factors & Formulas
Lattice Energy Lattice energy evaluates the intensity of the ionic …
Lattice Energy – Explanation, Factors & Formulas Read More »
Read More >>Lead Acetate – Definition, Properties, Uses
Lead Acetate Have you ever licked lipstick when you sketch …
Lead Acetate – Definition, Properties, Uses Read More »
Read More >>


















Comments: