Pi Bond
Orbitals are placed side by side where the electrons are placed heavily on the plane to form bonding atoms called pi bonds. Pi bonds are weaker than others since their negative charge atoms are present farther away from positively charged atoms and nuclei of the atom. According to quantum techniques, the p-orbitals are fragile due to the parallel structure. Its overlapping p-orbitals nature is the reason for its fragility of time present and considered the weaker atoms compared to others.
Let us see what are pi bonds, some of their characteristics, and the formation of their structure below for a detailed understanding.
What are Pi Bond Characteristics?
- A pi bond is formed on the orbital axis due to the overlapping structure of orbital atoms.
- Due to its parallel structure, it is brittle and fragile.
- A pi bond is formed from the structure of the sigma bond.
- A pi bond is not formed by saturated molecules as unsaturated molecules like alkanes and alkenes mainly include it.
- Pi bonds can form multiple bonds.
- It can’t form a single bond.
- It is not the prime bond in the information that is formed later.
- The pi bond decides the length of the molecule formations.
- It is less reactive to bond formations.
- Unsaturated molecules hold the pi bond mostly.
The pi bond definition is a naturally covalent chemical bond that involves the overlapping lateral structure of orbitals belonging to different atoms. The pi bond definition says that pi is related to the p orbitals as it has a parallel arrangement of atoms. D orbitals can also be placed in this bonding.
What are Pi Bond Formations?
As per the pi bond definition, two laterally or bonding sideways bonds are called lateral p orbitals, and the formation is called the pi orbital or covalent pi bond. If electrons heavily influence the axis bond on the p orbitals, it forms a pi bond.
The pi gets weak due to the partially lateral orbit formation. The pi becomes strong if the p orbitals have complete sideways overlapping and are parallel to each other. This is only reasonable if all of the atoms in the molecule are in the exact plane. This tells that the atom in the pi link does not rotate around the other atoms.
What is the Pi Bond Strength?
The Sigma bond is not that fragile like pi bonds and forms a particular strength to pi bonds. Considering the single c-c bond holds more strength than the double c-c bond that accounts for one pi and one sigma bond.
According to bond strength, it does not add fragility as a sigma bond as pi bonds have weak stability. Pi bond strength is measured in a quantum mechanical way and compared to the sigma bond strength.
So, as a result, the pi bond holds the lower degree of overlapping orbitals in its atom structure, resulting in a weaker bonding structure.
What is the Pi Bond structure in multiple bonds?
Pi bond, sigma bond, and delta bond are the three different structures of multiple bonds.
- The constant structure of the bond contains one pi bond and one sigma bond.
- The Eugene bond structure holds one pi and one sigma bond.
- The structure of three bonds contains two sigma bonds and one perpendicular axis atom on the orbitals.
- Bonds of four are rare to form and see.
- It is mainly seen in the axis that is present between atoms.
- It comprises one sigma, one delta, and two pi bonds.
Pi and sigma bonds combined to form the strongest bond structure compared to others.
What are the Pi Bond examples?
The pi bond example is Ethyne bonding, which illustrates pi bonding since it is coupled with one hydrogen atom and has threefold bonds between carbon particles.
The pi bond example bonds are generated due to the transfer of a particular wavelength of 2s electrons to 2pz. One 2s orbital will unify with one 2p orbital to generate the sigma pact and sp hybridization, while the remaining 2py and 2pz orbitals will create the pi bond.
Contemplate the ferrocene compound with two Fe- (C2H5) bonds. Ferrocene is coupled in a pi bond network because the chemical Fe is bound in a pi bond network.
What is the Pi Bond study?
Pi bonds occur between atoms in a molecule, not in the nooks of a regular tetrahedron. To analyze pi bonds, you must first grip the molecule’s geometry and its several varieties of bonding.
Molecular models can boost you in visualizing the contours of molecules and the stances of bonds. You can also utilize the molecular orbital hypothesis to comprehend molecule adhesion.
The importance of pi and sigma bonds is that pi bonds are elicited when two atoms stake two pairs of electrons, whereas sigma bonds are elicited when two atoms share one pair of electrons. Pi bonds are further reliable than sigma bonds.
What is the Pi Bond in an oxygen molecule?
The pi bond is generated when two atoms share a pair of electrons in a covalent pact. The two atoms in the oxygen molecule are the oxygen particles.
The two atoms deal with the electrons in the pi bond, and the bond is elicited by the extension of the atomic orbitals on the two atoms.
Atoms have distinct exposures in association with at least one another because covalent bonds appear to be directional. This provides accurate forms for the molecules, such as the angular structure of H2O molecules. Valence connections between identical molecules are nonpolar or electrically uniform, and those between non-uniform particles are polar or slightly negatively charged, meaning that the item that counts is slightly charged.
As the two atoms’ electronegativity increases, the valence bonds’ halfway ionic character will also increase. No molecules inside a compound have low ionization energy to cause electron loss when none of the components is metals. Valence thus wins out in such a situation. Valency bonds are typically created between pieces.
What is Pi Bond orbital overlapping?
This is the technique by which two atoms come so close that they infiltrate each other’s orbital and build a new hybrid.
The orbital in which the adhesion pair of electrons reside is known as orbital overlapping. The hybridized orbital is reliable and in its most profound energy state since it comprises less stability than the atomic orbital. This discriminatory penetration of the orbital is cited as orbital overlap.
The size and valence electrons of the two contributing atoms affect the degree of overlap. The bigger the overlap, the more powerful the bond formed between the two atoms. Atoms interact by coinciding with their orbitals, arising in a lower energy state in which their valence electrons with contrary spin cooperate to form a covalent bond.
What is Overlapping?
The procedure by which two atoms enter so close to each other that they enter each other’s orbital and induce a new hybridized orbital is known as orbital overlapping. Pi bonds are covalent chemical bonds formed by the lateral overlap of two lobes of one atomic orbital with two lobes of another atomic orbital from a different atom.
Pi bonds are created when orbitals cross laterally. They are created in a perpendicular direction when the sideways positive phase overlaps the internuclear axis within the same atomic orbital. During bond formation, the axes of the atomic orbitals are parallel to each other, whereas the overlapping is perpendicular to the internuclear axis. Due to its symmetric structure, the s orbital will always overlap axially with any other orbital.
Conclusion
Atoms are held concurrently by chemical bonds or holding troops. Valence, coordinate, ionic, and basic bonds are several sectors of chemical bonds. Bonds known as valence bonds are built when two atoms share an electron. They are also known as molecular bonds.
A chemical bond is an interatomic relationship that expands when two atoms share an electron. Their nuclei’s electrical affinity to identical electrons causes them to bond when specific atoms have less total stability than atoms with a large distance, and a chemical bond results.
Frequently asked questions
1, Why is the Pi bond weak and breaks fast?
The pi bond structure is formed from overlapping and side-by-side formations, making the bond more fragile and easy to break compared to other bond formations. The sigma bond formation is different from the pi bond, which makes it stronger.
2. Why are Pi bonds unstable?
The electrons present in the nuclei of an atom in a pi bond are far away from the positively charged atoms, and thus this structure makes the pi bond unstable and easier to break. The negatively charged atoms present near the electron of nuclei makethemt unstable in their bonding.
3. Which orbitals can form Pi bonds?
P-orbitals can form ponds since the orbital symmetry of the pi bond and p orbitals are the same, which can be seen uber the orbital bond axis. D-orbitals can also form the pi bond as it also has some of the same properties as the pi bond.

Relevant Articles
Butanoic Acid – Structure, Properties, Uses
Butanoic Acid The carboxylic acid, butanoic acid, has the structural …
Butanoic Acid – Structure, Properties, Uses Read More »
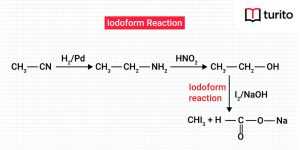
Read More >>What is Iodoform? Characteristics and Uses
Iodoform The formula for Iodoform is CHI3. It is biotic …
What is Iodoform? Characteristics and Uses Read More »
Read More >>Lattice Energy – Explanation, Factors & Formulas
Lattice Energy Lattice energy evaluates the intensity of the ionic …
Lattice Energy – Explanation, Factors & Formulas Read More »
Read More >>Lead Acetate – Definition, Properties, Uses
Lead Acetate Have you ever licked lipstick when you sketch …
Lead Acetate – Definition, Properties, Uses Read More »
Read More >>


















Comments: