Do you know what medicines were used in ancient times to treat diarrhoea? What was used popularly to dress skin burns? What was used for the treatment of unidentified abdominal disorders?
In history, there were no such medical facilities. Doctors used aerial plant tissues, tree bark, and herbs. Can you guess what chemical was present in aerial plant tissues that made their treatment successful?
This article is about tannic acid, a naturally arising plant polyphenol found in realistically all aerial plant tissues, which has a historical record in the treatment of the treatments mentioned above.
What Is Tannic Acid?
The simplest answer to the question of what is tannic acid it is a polyphenolic compound and is commercially available tannin. Although it is a form of natural tannins found in several plant groups, it is not a natural chemical. Tannins, the parent compound of tannic acid, are also found in coffee plants.
Tannic acid is based on the nutgalls manifested by insects on twigs of specific oak trees. It is removed from these twigs and used as medication. It is to be noted that coffee, tea and many other plants (notably tree bark and heartwoods) contain a lot of tannins, but most don’t contain tannic acid.
Types of Tannic Acid
Tannic acid has two classes:
1. Quercitannic acid:
It is found in both oak tree leaves and its bark.
2. Gallotannic acid:
It is found in the oak galls and a selected few other species.
Tannic Acid Structure
As tannic acid is a polyphenolic compound, its structure has numerous phenol groups. A particular tannin holds 10 galloyl (3,4,5-trihydroxyphenyl) units adjoining a glucose centre. However, the mercantile tannic acid structure consists of 2–12 half galloyl molecules. It comprises no carboxyl groups. But, due to the diversity of phenolic hydroxyls, it is weakly acidic.
The tannic acid structure is given below:

Properties of Tannic Acid
The properties of tannic acid are divided into physical and chemical categories. Some of them are:
1. Physical properties of tannic acid:
- The molecular formula of tannic acid is C₇₆H₅₂O₄₆.
- It is also known as Gallotannic acid or Gallotannin.
- The molecular weight of tannic acid is 1701.2 g/mol.
- It has a faint odour and is a light yellow to tan.
- Tannic acid exists in solid form.
- It has a density of 2.12g/cm³.
- The solubility of tannic acid in water is 250 g/L.
- It has an astringent taste.
- Tannic acid decomposes at 200°C.
- It sinks and mixes with water.
2. Chemical properties of tannic acid:
- It acts as a weak acid.
- Tannic acid molecules are unstable due to bacterial action and in the presence of oxygen and light.
- It does not react with iron. Hence, used as a corrosion inhibitor.
- It is insoluble in chloroform, benzene, carbon disulphide, diethyl ether, carbon tetrachloride (CCl₄), and petroleum.
- When heated, it decomposes to emit acrid smoke and fumes.
- It catalyses the formation of nitrosodiethylamine.
- The solution of tannic acid in glycerin is relatively stable.
The Function of Tannic Acid
Generally, tannic acid used for pharmaceutical actions is considered pentadigalloylglucose. It has a severe effect. Tannic acid is thought to exert antibacterial and antiviral effects.
When used internally, it dehydrates tissues and causes a reduction in secretions. Externally, it works by forming a protective layer of constricted and harder cells.
The main actions of tannic acid are because of its local effects. After ingestion, it shows a high affinity to plasma proteins, poor bioavailability because of its large size, and low lipid solubility.
What Is Tannic Acid Used For?
The following list below aids you in answering the question of what is tannic acid used for.
- Tannic acid has wide use in the food industry. Mostly it is operated to improve the clarity and taste of drinks.
- It does not contain any E number, which makes it a natural additive. Hence, it is highly used in beers, wine, and soft drinks.
- Because it produces dark natural colours, it is often used in wood stains.
- The dark colours of tannic acid assist in imitating light woods, the properties of dark ones.
- It is employed as aesthetic ether.
- The Sequoia redwoods use tannic acid and similar chemicals to protect against insects and wildfire. Otherwise, these things would eat into Sequoia’s heartwood.
- It is applied topically to treat diaper rash, cold sores, poison ivy, and fever blisters.
- It is used in both forms, i.e., orally and as ointments.
- In history, tannic acid was used as an antidote against different poisons.
- It is applied for chronic diarrhoea, bleeding, bloody urine, dysentery, persistent coughs, painful joints, and cancer.
- It can create a more stable form of oxidation layer on metals to stop rust from eating through the metal.
- It is operated in textiles to help colours stay fast when dyed, rendering fabrics stain-resistant.
What Are the Side Effects of Using Tannic Acid?
Although there are many uses of tannic acid, it can not be denied that there are some side effects of using it too. Some of them are
1. When taken by mouth:
In a certain amount, when tannic acid is taken through food items, it is likely safe. However, it can cause nausea, stomach irritation, and vomiting. Still, there is insufficient reliable information to verify that tannic acid is safe if used in larger medicinal amounts.
2. When applied to the skin:
Though applying tannic acid on minor burns or sunburns is fruitless, it is possibly unsafe to apply this chemical to tender or damaged skin. However, there is not enough genuine data that verifies whether using tannic acid on healthy and undamaged skin is safe or not.
What Are the Precautions Necessary to Take While Using Tannic Acid?
Some necessary precautions to take while using tannic acid are given below:
- It is unclear whether taking tannic acid orally during pregnancy and breastfeeding is safe. So, to be on the defence, prevent using it during these periods.
- It is unsafe to apply to broken skin or large areas of skin.
- It can cause kidney damage or worsen the kidney condition to an extreme. Hence, people having kidney disorders must avoid using it.
- It can cause liver damage. So, do not use tannic acid for those suffering from liver disorders.
- Do not cleanse yourself with added tannic acid if you have a problem with extensive skin damage and weeping eczema.
- If you have deep or large wounds, do not use tannic acid, as it gets into your body.
- If you have a heart failure problem or heart disease, do not take a bath after applying tannic acid.
- Do not take a bath with added tannic acid if you have a fever or infectious disease.
Conclusion
From the above article, you are now aware that tannic acid is found in the gallnut of certain oak trees. Insects form it on the twigs of these trees. Sometimes, purified tannic acid is used as medicine.
People use tannic acid for diaper rash, cold sores, heat rash, and many others. However, there are no adequate scientific proofs that support the use of tannic acid.
In beverages and foods, tannic acid is used as a flavouring agent. It is also operated in suppositories, ointments, tanning hides, manufacturing ink, and killing dust mites on furniture.
Frequently Asked Questions
1. What are the medicinal indications of tannic acid?
Tannic acid is indicated for fever blisters, cold sores, sunburn or minor burns, diaper rash, and prickly heat. Vaginally, it is used as a cleanser for leukorrhea. Tannic acid is also indicated for inflamed tonsils, sore throat, acute dermatitis, and receding or spongy gums.
2. What are the fruitless uses of tannic acid?
The places where tannic acid is ineffective to use are
- Diaper rash: On applying tannic acid, it does not seem to work on diaper rashes.
- Burns: Whether minor or sunburn, it does not seem to work on any burns.
- Heat rash: It does not seem to work on heat rashes too. So, it is a waste or fruitless to use tannic acid in this case.
- Cold sores (herpes labialis): It does not prove its importance or work in cold sore areas.
3. What are the acute effects of toxicity of tannic acid on humans?
Tannic acid is somewhat toxic by the ingestion and inhalation exposure pathways. Serious, high-dose absorption and ingestion may cause vomiting, nausea, abdominal pain, constipation, and liver damage. Acute intoxication may result in centrilobular liver necrosis.
4. What is the difference between tannin and tannic acid?
Tannins are polyphenolic biomolecules in the company of carbohydrate backbones. They are found in a wide scope of plants. Tannins are used in leather tanning. Other commercial uses are ink manufacture, paper sizing, dyeing, wine and food processing, and pyrogallol and gallic acid production.
Tannic acid is a particular tannin carrying 10 galloyl (3,4,5-trihydroxyphenyl) units. These galloyl units surround a glucose centre. It is a common mordant utilised in the dyeing procedure for cellulose fibres, after treatment to improve wash fastness, in the conservation of iron-based objects, etc.

Relevant Articles
Butanoic Acid – Structure, Properties, Uses
Butanoic Acid The carboxylic acid, butanoic acid, has the structural …
Butanoic Acid – Structure, Properties, Uses Read More »
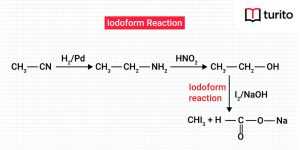
Read More >>What is Iodoform? Characteristics and Uses
Iodoform The formula for Iodoform is CHI3. It is biotic …
What is Iodoform? Characteristics and Uses Read More »
Read More >>Lattice Energy – Explanation, Factors & Formulas
Lattice Energy Lattice energy evaluates the intensity of the ionic …
Lattice Energy – Explanation, Factors & Formulas Read More »
Read More >>Lead Acetate – Definition, Properties, Uses
Lead Acetate Have you ever licked lipstick when you sketch …
Lead Acetate – Definition, Properties, Uses Read More »
Read More >>


















Comments: