The name “Zwitterion” originated from the German term “zwitter,” which is roughly comparable to “hermaphrodite” or “hybrid”. A zwitterion has two functional groups. They carry both positively and negatively charged electric ions. Consequently, zwitterions are largely electrically neutral (their net formal charge is generally zero).“Inner salts” is another name for zwitterions. Dipolar compounds are typically not categorized as zwitterions. The only difference is that the positive and negative signs on the amine oxide are used to indicate formal charges. Zwitterions may be worth considering in pharmacological design when working with acidic, basic, or neutral leads.
Zwitterion Definition
What is zwitterion? “A zwitterion can be defined as a molecule with both positive and negative charge areas.” In solid form, zwitterion amino acids persist as dipolar ions. When addressing whether a compound is zwitterionic, it is important to specify the pH range within which the data is required (since a properly alkaline solution will convert the zwitterion to an anion, and a properly acid solution will convert it to a cation).
The following is a list of several zwitterion properties:-
- Zwitterions are chemical compounds with positively and negatively charged ions, such as acid or base groupings. Ampholytes are a common example of a compound with both acid and basic groupings.
- The covalently bonding atoms hold the positively and negatively charged ions together in zwitterions.
- Since these compounds possess both positively and negatively charged electrical ions, they are also stable due to the unit electrical charges being kept apart from the atoms.
- Most zwitterions, such as those made by ammonium acids, have ammonium cations.
Zwitterion Amino Acid
Amino acids are the fundamental building blocks of proteins in all living cells. They are compounds made up of both carboxyl and amino groups. Proteins include twenty distinct amino acids. They have the same structure, where R stands for any of the 20 potential side chains on an amino acid.
The amino group (-NH3+) depicts a positive charge at neutral pH levels, while the carboxyl group (COO-) depicts a negative charge. Here is a picture of glycine, the most basic amino acid, in its zwitterion state:

Another intriguing feature of zwitterion amino acids is that they have no net charge if the molecule contains no other charged groups. Yes, a positive charge (+1) and a negative charge (-1) sum up to zero (neutral).
Except for amino acids, any chemical compound which contains acids and bases centres can generate a zwitterion form. Some of its examples are bicine, tricine, solid sulfamic acid, and alkaloids like psilocybin.
pH Value Affects Zwitterion Characteristics
A zwitterion isn’t necessarily a zwitterion, though. These compounds have neutral pH levels. Its groups can acquire various charges or become neutral at pH levels above or below 7. When the pH is low or acidic, the hydrogen ions combine with the carboxyl group to make it neutral. The amino acid now has a net charge of 1. The extra base at high or basic pH levels eliminates a hydrogen ion on N, neutralizing the amino group. As a result, giving the amino acid a net charge of -1.

Isoelectric Point
- There is an isoelectric point for each zwitterion (pI). The pH where a zwitterion is uncharged is known as the isoelectric point. The pH can modify a molecule’s charge by adding protons (H+).
- Since the amino group of an amino acid functions as a powerful proton acceptor, it is regarded as basic. The carboxyl group is regarded as acidic and works as a proton donor.
- The isoelectric point of a zwitterion amino acid can be determined by averaging the pKa values of the two functional groups. The functional group of each of the 20 distinct amino acids affects this (R group).
- The average isoelectric point of an amino acid is 5.5 because the mean pKa of the amino group is 9 and the mean pKa of the carboxyl group is 2. In other terms, amino acids typically have zero overall charges at a pH of 5.5.
Calculation of pH Value
The pH value at the isoelectric point can be computed from the zwitterion’s equilibrium constants (acids and bases). The formula is as follows;
pI = (pKa1 + pKa2)/ 2
Where,
pI = isoelectric point,
Ka1 = the equilibrium constant of the acids.
Ka2 = the equilibrium constant of the bases.
Application of Zwitterions
Zwitterionic compounds are featured with equal cation and anion groups on the molecular chains, which makes them extremely hydrophilic and antifouling. They can withstand the formation of bacterial adhesion, biofilms, and non-specific protein adsorption. They, therefore, offer a significant deal of promise for use in numerous biological and medically linked domains. Below are some of its typical applications:
SDS PAGE
Zwitterions have many helpful uses, but one of the most widespread practical techniques in molecular biology is SDS PAGE. This method of electrophoresis divides protein molecules according to their molecular weight. Glycine is one of the key elements of the mix used in SDS PAGE. The zwitterionic characteristics of glycine are crucial for this approach to protein separation. The pH of various SDS PAGE sections varies, which causes the glycine in the mix to have a different charge in various places. The charged and uncharged glycine is employed in combination with chloride ions present in the solution to ensure that all proteins form concentrated bands and move across the gel simultaneously.
Zwitterionic Polymers (compounds)
Long molecules known as zwitterionic polymers have functional groups that are both positively and negatively charged at either end. One key application is preventing biofilm development and microbial adhesion through their antifouling capabilities. Zwitterionic polymers are utilized in the marine sector to prevent the growth of subaquatic organisms on boats and piers. The antifouling capabilities of zwitterionic polymers are useful in two fields: the medical industry, drug delivery, medical implants and marine industries, to prevent the buildup of subaquatic organisms on boats and docks.
Uses in Medicine
In addition to their strong antifouling properties, zwitterionic materials have other advantages. They can increase biocompatibility, reduce immunological reactions, prolonged circulation time, and enable cellular uptake of traditional chemical medicines and therapeutic genes.
Informative detailsDue to their well-defined phase transformations in water solution, zwitterionic properties, and potential uses as ion exchange, complexation materials, catalysts, integrated drugs, and blood, macrocycles, protein stabilization, as well as electronic applications, zwitterionic polymers become the focus of both potential implementation and fundamental scientific experiments. |
Other Examples of Zwitterions
The majority of individuals first consider amino acids when they speak of zwitterions. However, other compounds can also have the same dipolar ion structure as amino acids. Sulfamic acid (NH3SO3) is not as well recognised as sulfuric acid (H2SO4) and sulphurous acid (H2SO3). It is used in cleaning goods as a substitute for hydrochloric acid because it is effective at eliminating lime and rust. Like zwitterion amino acids, nitrogen has a + charge, and oxygen has a – charge. Sometimes an extremely long molecule has the + and – groups at one end. Detergent-like compounds frequently contain them.

Things to Remember
- An active group molecule known as a zwitterion has at least one positive and negative electrical charge.
- The most appropriate example of zwitterion is an amino acid.
- Through electrostatic attraction with water molecules, zwitterions create a greater barrier to adsorption and strong hydration.
- Zwitterions do not travel in an electric field because they are electrically neutral.
- A zwitterion is frequently utilized when the protein molecules are separated using the SDS PAGE (sodium dodecyl sulphate-polyacrylamide gel electrophoresis) method.
Conclusion
Over the past few decades, significant volumes of innovative materials have been extensively investigated and created for industrial, electrical, biotechnological, and biological applications. One of these compounds that are now receiving a lot of attention in the field of biomaterials is zwitterionic polymers due to their extreme biocompatibility, hydrophilicity, and antifouling capabilities. Zwitterions are occasionally referred to as “inner salts.” Dipolar substances are typically not regarded as zwitterions. The positive and negative marks on the amine oxide signify formal charges. Zwitterions are useful in biopharmaceutical considerations when dealing with acid, basic, or neutral leads.
Frequently Asked Question on zwitterion
Q1.What is a zwitterion?
A:An ion that has two functional groups is called a zwitterion. Simply put, it is an ion that carries both positive and negative electrical charges. Zwitterions are largely electrically neutral (the net formal charge is mostly zero). Sometimes, zwitterions are referred to as “inner salts”
Q2.What is a zwitterion amino acid?
A:A molecule with both positive and negative ions is known as a zwitterion. The carboxylate ion provides the zwitterion amino acid with its negative charge, and the ammonium ion provides its positive charge.
Q3.How are zwitterions formed?
A:Zwitterions are dipolar ions formed by combining amino and carboxyl groups. As a result, an amino acid makes up the majority of it. In a zwitterion, the amine group has a positive charge, whereas the carboxyl group has a negative charge
Q4.Are zwitterions always neutral?
A:Due to their electrical neutrality, zwitterions do not move in an electric field. In an acidic medium (below pH level 2), the most common form of an amino acid is positively charged and flows towards the cathode.

Relevant Articles
Butanoic Acid – Structure, Properties, Uses
Butanoic Acid The carboxylic acid, butanoic acid, has the structural …
Butanoic Acid – Structure, Properties, Uses Read More »
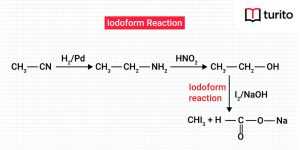
Read More >>What is Iodoform? Characteristics and Uses
Iodoform The formula for Iodoform is CHI3. It is biotic …
What is Iodoform? Characteristics and Uses Read More »
Read More >>Lattice Energy – Explanation, Factors & Formulas
Lattice Energy Lattice energy evaluates the intensity of the ionic …
Lattice Energy – Explanation, Factors & Formulas Read More »
Read More >>Lead Acetate – Definition, Properties, Uses
Lead Acetate Have you ever licked lipstick when you sketch …
Lead Acetate – Definition, Properties, Uses Read More »
Read More >>


















Comments: