Chemistry
Grade-9
Easy
Question
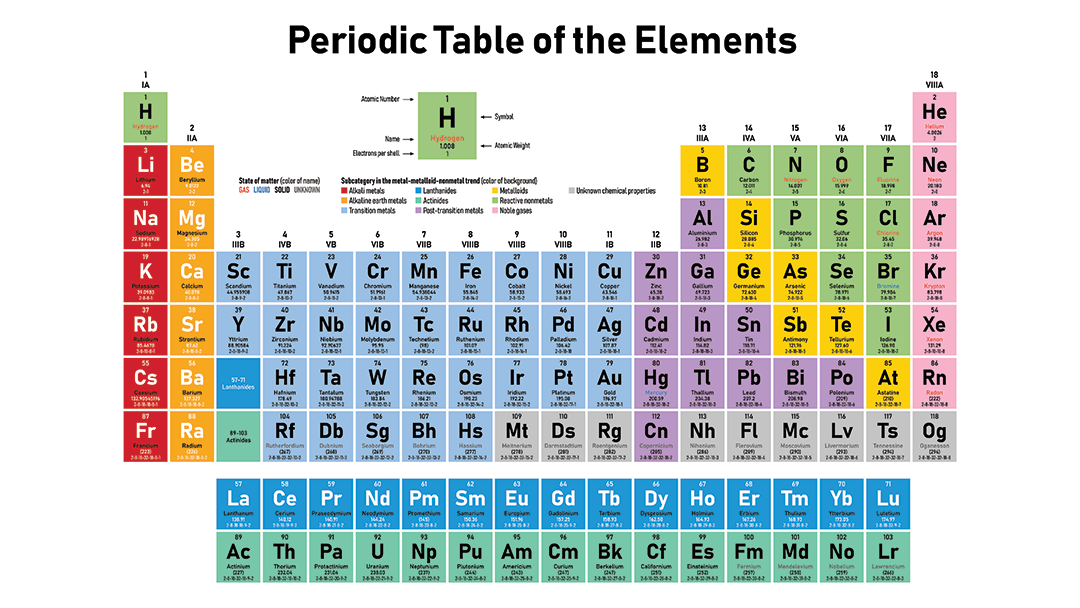
How would you describe reactivity on the Periodic Table?
- Elements in the same period tend to react.
- Elements in the same group tend to react.
- Elements on opposite sides of the table tend to react.
- All elements are inert.

Cations and anions tend to react.
The correct answer is: Elements on opposite sides of the table tend to react.
- Reactivity decrease as you go left to right of the periodic table, reactivity increases as you go down the group.
- Left side elements in the periodic table form cations and right side elements form anions.
- Elements on opposite sides of the table tend to react.
Related Questions to study
Chemistry
With which element will Hydrogen most likely bond?
With which element will Hydrogen most likely bond?

ChemistryGrade-9
Chemistry
Maximum covalency of Nitrogen is
Maximum covalency of Nitrogen is
ChemistryGrade-9
Chemistry
Electrons that involve in bond formation are
Electrons that involve in bond formation are
ChemistryGrade-9
Chemistry
All elements in the Halogen family react violently because
All elements in the Halogen family react violently because
ChemistryGrade-9
Chemistry
Where are the transition metals found on the periodic table?
Where are the transition metals found on the periodic table?
ChemistryGrade-9
Chemistry
How many elements are in the Noble Gas family?
How many elements are in the Noble Gas family?
ChemistryGrade-9
Chemistry

Chalcogens are

Chalcogens are
ChemistryGrade-9
Chemistry
Most reactive elemental gas.
Most reactive elemental gas.
ChemistryGrade-9
Chemistry

How many valence electrons are in the element Iodine?

How many valence electrons are in the element Iodine?
ChemistryGrade-9
Chemistry
Which of the statements on physical changes are correct?
Which of the statements on physical changes are correct?
ChemistryGrade-9
Chemistry
Coinage metals are:
Coinage metals are:
ChemistryGrade-9
Chemistry
A pure substance that is a compound is made out of
A pure substance that is a compound is made out of
ChemistryGrade-9
Chemistry
Match Transition metal to its right electronic configuration.

Match Transition metal to its right electronic configuration.

ChemistryGrade-9
Chemistry
Which of the following is the correct order in which the stability of the +2 oxidation state will exist for the four succeeding transition elements (Cr, Mn, Fe, and Co)?(At nos. Cr = 24, Mn = 25, Fe = 26, Co = 27)
Which of the following is the correct order in which the stability of the +2 oxidation state will exist for the four succeeding transition elements (Cr, Mn, Fe, and Co)?(At nos. Cr = 24, Mn = 25, Fe = 26, Co = 27)
ChemistryGrade-9





