Chemistry-
General
Easy
Question
Identify Y in the change;





The correct answer is: 
Related Questions to study
Chemistry-
The correct order of reactivity towards electrophilic substitution is
The correct order of reactivity towards electrophilic substitution is
Chemistry-General
Chemistry-
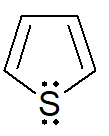
B basicity order of following heterocycles are
1) 
2) 
3) 
4) 
B basicity order of following heterocycles are
1) 
2) 
3) 
4) 
Chemistry-General
Chemistry-
Arrange the following in increasing order of electrophilic aromatic substitution.
I) 
II) 
III) 
IV) 
Arrange the following in increasing order of electrophilic aromatic substitution.
I) 
II) 
III) 
IV) 
Chemistry-General
Chemistry-
The order of the basicity in the following compounds is
I) 
II) 
III) 
IV) 
The order of the basicity in the following compounds is
I) 
II) 
III) 
IV) 
Chemistry-General
Chemistry-
Xylene on treatment with Br2/FeBr3 give only one product. Xylene was

Xylene on treatment with Br2/FeBr3 give only one product. Xylene was

Chemistry-General
Chemistry-
Which is true about the rate of nitration of the following compounds
I) 
II) 
Which is true about the rate of nitration of the following compounds
I) 
II) 
Chemistry-General
Chemistry-
Sandmeyer reaction involves the formation of
Sandmeyer reaction involves the formation of
Chemistry-General
Chemistry-
Arrange the following in increasing order of their activating capacity towards Bimolecular Aromatic substitution.
I) null,
II) CH3,
III) –NO2,
IV) null
Arrange the following in increasing order of their activating capacity towards Bimolecular Aromatic substitution.
I) null,
II) CH3,
III) –NO2,
IV) null
Chemistry-General
Chemistry-
Iodine Titration
All such titration which involves the direct titration of Iodine with a reducing agent are grouped under Iodimetry. Iodimetry is employed to determine the strength of reducing agent such as sodium those sulphate
I2 + Na2S2O3 ¾→ I– + null
If iodine is liberated as a result of chemical reaction involving oxidation of an idodide ion by a strong oxidizing agent in neutral or acidic medium the liberated iodine is then titrated with reducing agent. Iodometry is used to estimate the strength of oxidizing agent.
For example the estimation of Cu++ with thiosulphate.
Cu++ + I– ¾→Cu2I2 + I2
I2 + null
Starch used as indicator near the end point which form blue colour complex withnull. The blue colour disappears when there is no more of free I2.
100 ml of 0.1 N hypo decolourised iodine by the addition of x g of crystalline blue vitriol to excess of KI. The value of x is
Iodine Titration
All such titration which involves the direct titration of Iodine with a reducing agent are grouped under Iodimetry. Iodimetry is employed to determine the strength of reducing agent such as sodium those sulphate
I2 + Na2S2O3 ¾→ I– + null
If iodine is liberated as a result of chemical reaction involving oxidation of an idodide ion by a strong oxidizing agent in neutral or acidic medium the liberated iodine is then titrated with reducing agent. Iodometry is used to estimate the strength of oxidizing agent.
For example the estimation of Cu++ with thiosulphate.
Cu++ + I– ¾→Cu2I2 + I2
I2 + null
Starch used as indicator near the end point which form blue colour complex withnull. The blue colour disappears when there is no more of free I2.
100 ml of 0.1 N hypo decolourised iodine by the addition of x g of crystalline blue vitriol to excess of KI. The value of x is
Chemistry-General
Chemistry-
0.7 g of Na2CO3. xH2O is dissolved in 100 ml water, 20 ml of this solution required 19.8 ml of 0.1 N HCl. The value of x is
0.7 g of Na2CO3. xH2O is dissolved in 100 ml water, 20 ml of this solution required 19.8 ml of 0.1 N HCl. The value of x is
Chemistry-General
Chemistry-
0.1 g of bleaching powder, on reaction with acetic acid and excess KI solution, gave iodine which reacted with 50 ml of N/5 hypo. The per cent available Cl2 with sample is
0.1 g of bleaching powder, on reaction with acetic acid and excess KI solution, gave iodine which reacted with 50 ml of N/5 hypo. The per cent available Cl2 with sample is
Chemistry-General
Chemistry-
Calculate the weight of ion which will be converted into its oxide by the action of 18g of steam on it.
Calculate the weight of ion which will be converted into its oxide by the action of 18g of steam on it.
Chemistry-General
Chemistry-
An aqueous solution of 6.3 gm oxalic acid dihydrate is made upto 250 ml. The volume of 0.1 N NaOH required to completely neutralise 10 ml of this solution is
An aqueous solution of 6.3 gm oxalic acid dihydrate is made upto 250 ml. The volume of 0.1 N NaOH required to completely neutralise 10 ml of this solution is
Chemistry-General
Chemistry-
In the modern periodic table, elements are arranged in order of increasing atomic number and is based upon the electronic configuration of elements. Depending upon the type of subshell which receives the last electron, the elements in the periodic table have been divided into four blocks, viz., s–, p–, d– and f–. The modern periodic table consisits of 7 periods and 18 groups. Each period beings with the filling of a new energy shell. In accordance with the aufbau principle, the seven periods have 2, 8, 8, 18, 18, 32 and 32 elements respectively. The seventh period still incomplete. To avoid making the period table too long, the two series of f–block elements called lanthanoids and actinoids are placed at the bottom of the main body of the periodic table.
Identify the least stable ion amongest of the following :
In the modern periodic table, elements are arranged in order of increasing atomic number and is based upon the electronic configuration of elements. Depending upon the type of subshell which receives the last electron, the elements in the periodic table have been divided into four blocks, viz., s–, p–, d– and f–. The modern periodic table consisits of 7 periods and 18 groups. Each period beings with the filling of a new energy shell. In accordance with the aufbau principle, the seven periods have 2, 8, 8, 18, 18, 32 and 32 elements respectively. The seventh period still incomplete. To avoid making the period table too long, the two series of f–block elements called lanthanoids and actinoids are placed at the bottom of the main body of the periodic table.
Identify the least stable ion amongest of the following :
Chemistry-General
Physics-
The acceleration of a particle is increasing linearly with time t as b t The particle starts from the origin with an initial velocity  The distance travelled by the particle in time t will be :
The distance travelled by the particle in time t will be :
The acceleration of a particle is increasing linearly with time t as b t The particle starts from the origin with an initial velocity  The distance travelled by the particle in time t will be :
The distance travelled by the particle in time t will be :
Physics-General





