Chemistry-
General
Easy
Question
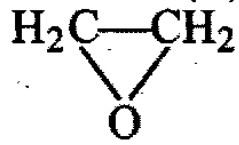
In this reaction,

the molecule (A) and the reagent (B) are :
 hot water
hot water



 hot water
hot water
The correct answer is: 
Related Questions to study
Chemistry-
Propene on reaction with carbon monoxide and hydrogen at high temperature under pressure in presence of cobalt carbonyl catalyst gives:
Propene on reaction with carbon monoxide and hydrogen at high temperature under pressure in presence of cobalt carbonyl catalyst gives:
Chemistry-General
Chemistry-
What is the product (A) in the following reaction?

What is the product (A) in the following reaction?

Chemistry-General
Chemistry-
A compound soluble in conc.  ' It does not decolourise bromine in
' It does not decolourise bromine in  but oxidised by chromic anhydride in aqueous
but oxidised by chromic anhydride in aqueous  within two seconds, turning orange solution to blue, green, and then opaque. The original compound is:
within two seconds, turning orange solution to blue, green, and then opaque. The original compound is:
A compound soluble in conc.  ' It does not decolourise bromine in
' It does not decolourise bromine in  but oxidised by chromic anhydride in aqueous
but oxidised by chromic anhydride in aqueous  within two seconds, turning orange solution to blue, green, and then opaque. The original compound is:
within two seconds, turning orange solution to blue, green, and then opaque. The original compound is:
Chemistry-General
Chemistry-
Free radical halogenation takes place in the presence of light or at high temperature (above 773K). Formation of halogen free radical intermediate takes place in first step called chain initiation step.

This reaction is mainly given by those compounds which have atleast one hydrogen atom present at  -hybrid carbon. Reactivity of
-hybrid carbon. Reactivity of  -hybrid carbon depends on the reactivity of reaction intermediate. The relative rate of formation of alkyl radicals by a chlorine radical is :
-hybrid carbon depends on the reactivity of reaction intermediate. The relative rate of formation of alkyl radicals by a chlorine radical is :

Percentage yield of the product =
Relative amount = Number of hydrogen atoms on the respective carbon x relative reactivity.
NBS (N-bromo succinimide) is used for bromination at allylic and benzylic carbon, whereas Br2/hv gives bromination at . benzylic, allylic and alkyl carbon.
Arrange decreasing order of reactivity of given compounds with NBS: (N-bromo succinimide)
I) 
II) 
III) 
IV) 
Select the correct answer from the codes given below:
Free radical halogenation takes place in the presence of light or at high temperature (above 773K). Formation of halogen free radical intermediate takes place in first step called chain initiation step.

This reaction is mainly given by those compounds which have atleast one hydrogen atom present at  -hybrid carbon. Reactivity of
-hybrid carbon. Reactivity of  -hybrid carbon depends on the reactivity of reaction intermediate. The relative rate of formation of alkyl radicals by a chlorine radical is :
-hybrid carbon depends on the reactivity of reaction intermediate. The relative rate of formation of alkyl radicals by a chlorine radical is :

Percentage yield of the product =
Relative amount = Number of hydrogen atoms on the respective carbon x relative reactivity.
NBS (N-bromo succinimide) is used for bromination at allylic and benzylic carbon, whereas Br2/hv gives bromination at . benzylic, allylic and alkyl carbon.
Arrange decreasing order of reactivity of given compounds with NBS: (N-bromo succinimide)
I) 
II) 
III) 
IV) 
Select the correct answer from the codes given below:
Chemistry-General
Chemistry-
Free radical halogenation takes place in the presence of light or at high temperature (above 773K). Formation of halogen free radical intermediate takes place in first step called chain initiation step.

This reaction is mainly given by those compounds which have atleast one hydrogen atom present at  -hybrid carbon. Reactivity of
-hybrid carbon. Reactivity of  -hybrid carbon depends on the reactivity of reaction intermediate. The relative rate of formation of alkyl radicals by a chlorine radical is :
-hybrid carbon depends on the reactivity of reaction intermediate. The relative rate of formation of alkyl radicals by a chlorine radical is :

Percentage yield of the product =
Relative amount = Number of hydrogen atoms on the respective carbon x relative reactivity.
NBS (N-bromo succinimide) is used for bromination at allylic and benzylic carbon, whereas Br2/hv gives bromination at . benzylic, allylic and alkyl carbon.
Chlorination of butane takes place as:

Percentage yield of 2-chlorobutane will be:
Free radical halogenation takes place in the presence of light or at high temperature (above 773K). Formation of halogen free radical intermediate takes place in first step called chain initiation step.

This reaction is mainly given by those compounds which have atleast one hydrogen atom present at  -hybrid carbon. Reactivity of
-hybrid carbon. Reactivity of  -hybrid carbon depends on the reactivity of reaction intermediate. The relative rate of formation of alkyl radicals by a chlorine radical is :
-hybrid carbon depends on the reactivity of reaction intermediate. The relative rate of formation of alkyl radicals by a chlorine radical is :

Percentage yield of the product =
Relative amount = Number of hydrogen atoms on the respective carbon x relative reactivity.
NBS (N-bromo succinimide) is used for bromination at allylic and benzylic carbon, whereas Br2/hv gives bromination at . benzylic, allylic and alkyl carbon.
Chlorination of butane takes place as:

Percentage yield of 2-chlorobutane will be:
Chemistry-General
Chemistry-
SN1 reaction is a first order nucleophilic substitution, e.g.,

The concentration of nucleophile does not appear in the rate law expression:
Reaction rate = k[ RX]
In a multistep organic reaction, the rate-limiting step is the slowest step. Rate determining step is represented by the following energy level diagram:


A reaction energy level diagram for an  reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate
reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate

 reaction is:
reaction is:
SN1 reaction is a first order nucleophilic substitution, e.g.,

The concentration of nucleophile does not appear in the rate law expression:
Reaction rate = k[ RX]
In a multistep organic reaction, the rate-limiting step is the slowest step. Rate determining step is represented by the following energy level diagram:


A reaction energy level diagram for an  reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate
reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate

 reaction is:
reaction is:
Chemistry-General
Chemistry-
SN1 reaction is a first order nucleophilic substitution, e.g.,

The concentration of nucleophile does not appear in the rate law expression:
Reaction rate = k[ RX]
In a multistep organic reaction, the rate-limiting step is the slowest step. Rate determining step is represented by the following energy level diagram:


A reaction energy level diagram for an  reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate
reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate

In the graph 3 for  reaction, the rate limiting step is the spontaneous dissociation of alkyl halide and is given by:
reaction, the rate limiting step is the spontaneous dissociation of alkyl halide and is given by:
SN1 reaction is a first order nucleophilic substitution, e.g.,

The concentration of nucleophile does not appear in the rate law expression:
Reaction rate = k[ RX]
In a multistep organic reaction, the rate-limiting step is the slowest step. Rate determining step is represented by the following energy level diagram:


A reaction energy level diagram for an  reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate
reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate

In the graph 3 for  reaction, the rate limiting step is the spontaneous dissociation of alkyl halide and is given by:
reaction, the rate limiting step is the spontaneous dissociation of alkyl halide and is given by:
Chemistry-General
Chemistry-
SN1 reaction is a first order nucleophilic substitution, e.g.,

The concentration of nucleophile does not appear in the rate law expression:
Reaction rate = k[ RX]
In a multistep organic reaction, the rate-limiting step is the slowest step. Rate determining step is represented by the following energy level diagram:


A reaction energy level diagram for an  reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate
reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate

Select the correct statement(s) about the graph 2:
SN1 reaction is a first order nucleophilic substitution, e.g.,

The concentration of nucleophile does not appear in the rate law expression:
Reaction rate = k[ RX]
In a multistep organic reaction, the rate-limiting step is the slowest step. Rate determining step is represented by the following energy level diagram:


A reaction energy level diagram for an  reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate
reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate

Select the correct statement(s) about the graph 2:
Chemistry-General
Chemistry-
SN1 reaction is a first order nucleophilic substitution, e.g.,

The concentration of nucleophile does not appear in the rate law expression:
Reaction rate = k[ RX]
In a multistep organic reaction, the rate-limiting step is the slowest step. Rate determining step is represented by the following energy level diagram:


A reaction energy level diagram for an  reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate
reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate

In  reaction, the hybridization changes in rate determination step. Select the correct change among the following:
reaction, the hybridization changes in rate determination step. Select the correct change among the following:
SN1 reaction is a first order nucleophilic substitution, e.g.,

The concentration of nucleophile does not appear in the rate law expression:
Reaction rate = k[ RX]
In a multistep organic reaction, the rate-limiting step is the slowest step. Rate determining step is represented by the following energy level diagram:


A reaction energy level diagram for an  reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate
reaction. The rate limiting step is spontaneous dissociation of an alkyl halide to give a carbocation intermediate

In  reaction, the hybridization changes in rate determination step. Select the correct change among the following:
reaction, the hybridization changes in rate determination step. Select the correct change among the following:
Chemistry-General
Chemistry-
The removal of two atoms or groups, one generally hydrogen  and the other a leaving group
and the other a leaving group  resulting in the formation of unsaturated compound is known as elimination reaction.
resulting in the formation of unsaturated compound is known as elimination reaction.

In  (elimination) reactions, the
(elimination) reactions, the  bond is broken heterolytic ally (in step 1) to form a carbocation (as in
bond is broken heterolytic ally (in step 1) to form a carbocation (as in  reaction) in which
reaction) in which  ) is lost (rate determining step). The carbocation (in step 2) loses a proton from the
) is lost (rate determining step). The carbocation (in step 2) loses a proton from the  -carbon atom by a base (nucleophile) to form an alkene.
-carbon atom by a base (nucleophile) to form an alkene.  reaction is favoured in compounds in which the leaving group is at secondary
reaction is favoured in compounds in which the leaving group is at secondary  or tertiary
or tertiary  position. In
position. In  formed simultaneously.
formed simultaneously.  reactions occur in one step through a transition state.
reactions occur in one step through a transition state.

 reactions are most common in haloalkanes (particularly,
reactions are most common in haloalkanes (particularly,  ) and better the leaving group higher is the
) and better the leaving group higher is the  reaction. In
reaction. In  reactions, both the leaving groups should be antiplanar.
reactions, both the leaving groups should be antiplanar.
 cb (Elimination unimolecular conjugate base) reaction involves the removal of proton by. a conjugate base (step 1) to produce carbanion which loses a leaving group to form an alkene (step 2) and is a slow step.
cb (Elimination unimolecular conjugate base) reaction involves the removal of proton by. a conjugate base (step 1) to produce carbanion which loses a leaving group to form an alkene (step 2) and is a slow step.
Neopentyl bromide undergoes dehydrohalogenation to give alkene even though it has no  -hydrogen. This is due to:
-hydrogen. This is due to:
The removal of two atoms or groups, one generally hydrogen  and the other a leaving group
and the other a leaving group  resulting in the formation of unsaturated compound is known as elimination reaction.
resulting in the formation of unsaturated compound is known as elimination reaction.

In  (elimination) reactions, the
(elimination) reactions, the  bond is broken heterolytic ally (in step 1) to form a carbocation (as in
bond is broken heterolytic ally (in step 1) to form a carbocation (as in  reaction) in which
reaction) in which  ) is lost (rate determining step). The carbocation (in step 2) loses a proton from the
) is lost (rate determining step). The carbocation (in step 2) loses a proton from the  -carbon atom by a base (nucleophile) to form an alkene.
-carbon atom by a base (nucleophile) to form an alkene.  reaction is favoured in compounds in which the leaving group is at secondary
reaction is favoured in compounds in which the leaving group is at secondary  or tertiary
or tertiary  position. In
position. In  formed simultaneously.
formed simultaneously.  reactions occur in one step through a transition state.
reactions occur in one step through a transition state.

 reactions are most common in haloalkanes (particularly,
reactions are most common in haloalkanes (particularly,  ) and better the leaving group higher is the
) and better the leaving group higher is the  reaction. In
reaction. In  reactions, both the leaving groups should be antiplanar.
reactions, both the leaving groups should be antiplanar.
 cb (Elimination unimolecular conjugate base) reaction involves the removal of proton by. a conjugate base (step 1) to produce carbanion which loses a leaving group to form an alkene (step 2) and is a slow step.
cb (Elimination unimolecular conjugate base) reaction involves the removal of proton by. a conjugate base (step 1) to produce carbanion which loses a leaving group to form an alkene (step 2) and is a slow step.
Neopentyl bromide undergoes dehydrohalogenation to give alkene even though it has no  -hydrogen. This is due to:
-hydrogen. This is due to:
Chemistry-General
Chemistry-
The removal of two atoms or groups, one generally hydrogen  and the other a leaving group
and the other a leaving group  resulting in the formation of unsaturated compound is known as elimination reaction.
resulting in the formation of unsaturated compound is known as elimination reaction.

In  (elimination) reactions, the
(elimination) reactions, the  bond is broken heterolytic ally (in step 1) to form a carbocation (as in
bond is broken heterolytic ally (in step 1) to form a carbocation (as in  reaction) in which
reaction) in which  ) is lost (rate determining step). The carbocation (in step 2) loses a proton from the
) is lost (rate determining step). The carbocation (in step 2) loses a proton from the  -carbon atom by a base (nucleophile) to form an alkene.
-carbon atom by a base (nucleophile) to form an alkene.  reaction is favoured in compounds in which the leaving group is at secondary
reaction is favoured in compounds in which the leaving group is at secondary  or tertiary
or tertiary  position. In
position. In  formed simultaneously.
formed simultaneously.  reactions occur in one step through a transition state.
reactions occur in one step through a transition state.

 reactions are most common in haloalkanes (particularly,
reactions are most common in haloalkanes (particularly,  ) and better the leaving group higher is the
) and better the leaving group higher is the  reaction. In
reaction. In  reactions, both the leaving groups should be antiplanar.
reactions, both the leaving groups should be antiplanar.
 cb (Elimination unimolecular conjugate base) reaction involves the removal of proton by. a conjugate base (step 1) to produce carbanion which loses a leaving group to form an alkene (step 2) and is a slow step.
cb (Elimination unimolecular conjugate base) reaction involves the removal of proton by. a conjugate base (step 1) to produce carbanion which loses a leaving group to form an alkene (step 2) and is a slow step.
2-Br-omopentane is heated with potassium ethoxide in ethanol. The major product obtained is:
The removal of two atoms or groups, one generally hydrogen  and the other a leaving group
and the other a leaving group  resulting in the formation of unsaturated compound is known as elimination reaction.
resulting in the formation of unsaturated compound is known as elimination reaction.

In  (elimination) reactions, the
(elimination) reactions, the  bond is broken heterolytic ally (in step 1) to form a carbocation (as in
bond is broken heterolytic ally (in step 1) to form a carbocation (as in  reaction) in which
reaction) in which  ) is lost (rate determining step). The carbocation (in step 2) loses a proton from the
) is lost (rate determining step). The carbocation (in step 2) loses a proton from the  -carbon atom by a base (nucleophile) to form an alkene.
-carbon atom by a base (nucleophile) to form an alkene.  reaction is favoured in compounds in which the leaving group is at secondary
reaction is favoured in compounds in which the leaving group is at secondary  or tertiary
or tertiary  position. In
position. In  formed simultaneously.
formed simultaneously.  reactions occur in one step through a transition state.
reactions occur in one step through a transition state.

 reactions are most common in haloalkanes (particularly,
reactions are most common in haloalkanes (particularly,  ) and better the leaving group higher is the
) and better the leaving group higher is the  reaction. In
reaction. In  reactions, both the leaving groups should be antiplanar.
reactions, both the leaving groups should be antiplanar.
 cb (Elimination unimolecular conjugate base) reaction involves the removal of proton by. a conjugate base (step 1) to produce carbanion which loses a leaving group to form an alkene (step 2) and is a slow step.
cb (Elimination unimolecular conjugate base) reaction involves the removal of proton by. a conjugate base (step 1) to produce carbanion which loses a leaving group to form an alkene (step 2) and is a slow step.
2-Br-omopentane is heated with potassium ethoxide in ethanol. The major product obtained is:
Chemistry-General
Chemistry-
The removal of two atoms or groups, one generally hydrogen  and the other a leaving group
and the other a leaving group  resulting in the formation of unsaturated compound is known as elimination reaction.
resulting in the formation of unsaturated compound is known as elimination reaction.

In  (elimination) reactions, the
(elimination) reactions, the  bond is broken heterolytic ally (in step 1) to form a carbocation (as in
bond is broken heterolytic ally (in step 1) to form a carbocation (as in  reaction) in which
reaction) in which  ) is lost (rate determining step). The carbocation (in step 2) loses a proton from the
) is lost (rate determining step). The carbocation (in step 2) loses a proton from the  -carbon atom by a base (nucleophile) to form an alkene.
-carbon atom by a base (nucleophile) to form an alkene.  reaction is favoured in compounds in which the leaving group is at secondary
reaction is favoured in compounds in which the leaving group is at secondary  or tertiary
or tertiary  position. In
position. In  formed simultaneously.
formed simultaneously.  reactions occur in one step through a transition state.
reactions occur in one step through a transition state.

 reactions are most common in haloalkanes (particularly,
reactions are most common in haloalkanes (particularly,  ) and better the leaving group higher is the
) and better the leaving group higher is the  reaction. In
reaction. In  reactions, both the leaving groups should be antiplanar.
reactions, both the leaving groups should be antiplanar.
 cb (Elimination unimolecular conjugate base) reaction involves the removal of proton by. a conjugate base (step 1) to produce carbanion which loses a leaving group to form an alkene (step 2) and is a slow step.
cb (Elimination unimolecular conjugate base) reaction involves the removal of proton by. a conjugate base (step 1) to produce carbanion which loses a leaving group to form an alkene (step 2) and is a slow step.

This reaction is an example of:
The removal of two atoms or groups, one generally hydrogen  and the other a leaving group
and the other a leaving group  resulting in the formation of unsaturated compound is known as elimination reaction.
resulting in the formation of unsaturated compound is known as elimination reaction.

In  (elimination) reactions, the
(elimination) reactions, the  bond is broken heterolytic ally (in step 1) to form a carbocation (as in
bond is broken heterolytic ally (in step 1) to form a carbocation (as in  reaction) in which
reaction) in which  ) is lost (rate determining step). The carbocation (in step 2) loses a proton from the
) is lost (rate determining step). The carbocation (in step 2) loses a proton from the  -carbon atom by a base (nucleophile) to form an alkene.
-carbon atom by a base (nucleophile) to form an alkene.  reaction is favoured in compounds in which the leaving group is at secondary
reaction is favoured in compounds in which the leaving group is at secondary  or tertiary
or tertiary  position. In
position. In  formed simultaneously.
formed simultaneously.  reactions occur in one step through a transition state.
reactions occur in one step through a transition state.

 reactions are most common in haloalkanes (particularly,
reactions are most common in haloalkanes (particularly,  ) and better the leaving group higher is the
) and better the leaving group higher is the  reaction. In
reaction. In  reactions, both the leaving groups should be antiplanar.
reactions, both the leaving groups should be antiplanar.
 cb (Elimination unimolecular conjugate base) reaction involves the removal of proton by. a conjugate base (step 1) to produce carbanion which loses a leaving group to form an alkene (step 2) and is a slow step.
cb (Elimination unimolecular conjugate base) reaction involves the removal of proton by. a conjugate base (step 1) to produce carbanion which loses a leaving group to form an alkene (step 2) and is a slow step.

This reaction is an example of:
Chemistry-General
Maths-
For any real x, the expression  cannot exceed
cannot exceed
For any real x, the expression  cannot exceed
cannot exceed
Maths-General
Maths-
The number of real roots of  is
is
The number of real roots of  is
is
Maths-General
Maths-
If z1, z2, z3 are complex numbers such that∣z1∣=∣ z2 ∣=∣ z3 ∣= , thus, ∣ z1 + z2 + z3 ∣ is
, thus, ∣ z1 + z2 + z3 ∣ is
If z1, z2, z3 are complex numbers such that∣z1∣=∣ z2 ∣=∣ z3 ∣= , thus, ∣ z1 + z2 + z3 ∣ is
, thus, ∣ z1 + z2 + z3 ∣ is
Maths-General





