Chemistry-
General
Easy
Question
Oxidation without cleavage of sigma bond takes place in alkenes.

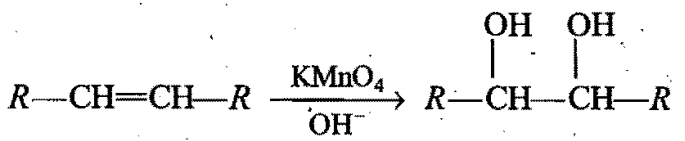
Presence of unsaturation· -in alkenes is detected by using Baeyer's reagent. Alkenes decolourise pink colour of Baeyer’s. reagent. In presence of Baeyer's reagent, 'syn' addition of-OH groups takes place on both carbons· of double· bond. The net reaction can be given as,
Ozonolysis of· alkenes give ozonide, which on further hydrolysis gives aldehyde and/or ketone.

Linear polyenes on. ozonolysis gives two moles of acetaldehyde and one mole of propanedial. Linear polyene will be:
- alkadiene
- alkatriene
- alkatetraene
- alkapentaene
The correct answer is: alkadiene
Related Questions to study
Chemistry-
Hydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the. addition ·of hydrogen takes·· place in presence of Raney nickel· catalyst. Platinum and palladium can also be used as catalyst in these reactions. These are heterogeneous catalyst and used in. finely. divided state. Experimentally, it is observed that less. crowded alkenes adsorb H2 with faster rate. Controlled hydrogeneration of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:

Non-terminal alkynes an reduce4 in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product. .
The product of the following reaction is:
<img src="https://mycourses.turito.com/tokenpluginfile.php/c161933dbfaab094c54655ab71e9b8f0/1/question/questiontext/649779/1/1178377/Picture21.png" alt="" width="285" height="89"
Hydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the. addition ·of hydrogen takes·· place in presence of Raney nickel· catalyst. Platinum and palladium can also be used as catalyst in these reactions. These are heterogeneous catalyst and used in. finely. divided state. Experimentally, it is observed that less. crowded alkenes adsorb H2 with faster rate. Controlled hydrogeneration of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:

Non-terminal alkynes an reduce4 in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product. .
The product of the following reaction is:
<img src="https://mycourses.turito.com/tokenpluginfile.php/c161933dbfaab094c54655ab71e9b8f0/1/question/questiontext/649779/1/1178377/Picture21.png" alt="" width="285" height="89"
Chemistry-General
Chemistry-
Hydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the. addition ·of hydrogen takes·· place in presence of Raney nickel· catalyst. Platinum and palladium can also be used as catalyst in these reactions. These are heterogeneous catalyst and used in. finely. divided state. Experimentally, it is observed that less. crowded alkenes adsorb H2 with faster rate. Controlled hydrogeneration of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:

Non-terminal alkynes an reduce4 in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product.
In which of the following cases, the reaction is most. exothermic?
Hydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the. addition ·of hydrogen takes·· place in presence of Raney nickel· catalyst. Platinum and palladium can also be used as catalyst in these reactions. These are heterogeneous catalyst and used in. finely. divided state. Experimentally, it is observed that less. crowded alkenes adsorb H2 with faster rate. Controlled hydrogeneration of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:

Non-terminal alkynes an reduce4 in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product.
In which of the following cases, the reaction is most. exothermic?
Chemistry-General
Chemistry-
Hydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the. addition ·of hydrogen takes·· place in presence of Raney nickel· catalyst. Platinum and palladium can also be used as catalyst in these reactions. These are heterogeneous catalyst and used in. finely. divided state. Experimentally, it is observed that less. crowded alkenes adsorb H2 with faster rate. Controlled hydrogeneration of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:

Non-terminal alkynes a reduce4 in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product.
The relative rate of catalytic hydrogenation of following. alkenes are:
I) 
II) 
III) 
IV)  </span
</span
Hydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the. addition ·of hydrogen takes·· place in presence of Raney nickel· catalyst. Platinum and palladium can also be used as catalyst in these reactions. These are heterogeneous catalyst and used in. finely. divided state. Experimentally, it is observed that less. crowded alkenes adsorb H2 with faster rate. Controlled hydrogeneration of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:

Non-terminal alkynes a reduce4 in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product.
The relative rate of catalytic hydrogenation of following. alkenes are:
I) 
II) 
III) 
IV)  </span
</span
Chemistry-General
Chemistry-
 Compounds [X] and [Y] are respectively:
Compounds [X] and [Y] are respectively:
 Compounds [X] and [Y] are respectively:
Compounds [X] and [Y] are respectively:
Chemistry-General
Chemistry-
Unknown Compound (A) on oxidation with hot basic. KMnO4 'gives only one compound whose structure is given below,

Compound (A) will be:
Unknown Compound (A) on oxidation with hot basic. KMnO4 'gives only one compound whose structure is given below,

Compound (A) will be:
Chemistry-General
Chemistry-
Order of reactivity of given four alkenes for hydrogenation , reaction will be:
I) 
II) 
III) 
IV) 
Order of reactivity of given four alkenes for hydrogenation , reaction will be:
I) 
II) 
III) 
IV) 
Chemistry-General
Maths-
A pie chart is to be drawn for representing the following data The value of the central angle for food and clothing would be

A pie chart is to be drawn for representing the following data The value of the central angle for food and clothing would be

Maths-General
Maths-
The following data gives the distribution of height of students The median of the distribution is

The following data gives the distribution of height of students The median of the distribution is

Maths-General
Maths-
The quartile deviation for the following data is

The quartile deviation for the following data is

Maths-General
Maths-
Section-wise expenditure of a State Govt. is shown in the given figure. The expenditure incurred on transport is

Section-wise expenditure of a State Govt. is shown in the given figure. The expenditure incurred on transport is

Maths-General
Maths-
A market with 3900 operating firms has the following distribution for firms arranged according to various income groups of workers If a histogram for the above distribution is constructed the highest bar in the histogram would correspond to the class

A market with 3900 operating firms has the following distribution for firms arranged according to various income groups of workers If a histogram for the above distribution is constructed the highest bar in the histogram would correspond to the class

Maths-General
Maths-
The mortality in a town during 4 quarters of a year due to various causes is given below: Based on this data, the percentage increase in mortality in the third quarter is

The mortality in a town during 4 quarters of a year due to various causes is given below: Based on this data, the percentage increase in mortality in the third quarter is

Maths-General
Maths-
The following data was collected from the newspaper: (percentage distribution)It is an example of

The following data was collected from the newspaper: (percentage distribution)It is an example of

Maths-General
Maths-
The mode of the distribution

The mode of the distribution

Maths-General
Maths-
The upper quartile for the following distribution is given by the size of

The upper quartile for the following distribution is given by the size of

Maths-General



