Chemistry-
General
Easy
Question
 reaction is favoured by:
reaction is favoured by:
- bulky groups on the carbon atom attached to halogen atom '
- small groups on the carbon atom attached to halogen atom,
- non-polar solvents
- none of the above
The correct answer is: bulky groups on the carbon atom attached to halogen atom '
Related Questions to study
Chemistry-
Order of base strength of the compounds :
I) 
II) 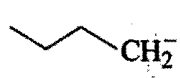
III) 
IV) 
Order of base strength of the compounds :
I) 
II) 
III) 
IV) 
Chemistry-General
Chemistry-
The stability of the following carbocation decreases in the order:
I) 
II) 
III) 
IV) 
The stability of the following carbocation decreases in the order:
I) 
II) 
III) 
IV) 
Chemistry-General
Chemistry-
Which of the following orders is correct for the ease of electrophile addition on these alkenes?
I) 
II) 
III) 
Which of the following orders is correct for the ease of electrophile addition on these alkenes?
I) 
II) 
III) 
Chemistry-General
Chemistry-
 H for CaCO3(s)
H for CaCO3(s)  CaO(s) + CO2(g) is 176 kJ mol–1 at 1240 K. The
CaO(s) + CO2(g) is 176 kJ mol–1 at 1240 K. The  E for the change is equal to
E for the change is equal to
 H for CaCO3(s)
H for CaCO3(s)  CaO(s) + CO2(g) is 176 kJ mol–1 at 1240 K. The
CaO(s) + CO2(g) is 176 kJ mol–1 at 1240 K. The  E for the change is equal to
E for the change is equal to
Chemistry-General
Chemistry-
The heat evolved in combustion if 112 litres of water gas (mixture of equal volume of H2(g) and CO(g) is
H2(g) + 1/2 O2(g) = H2O(g)  H = –241.8 kJ
H = –241.8 kJ
CO(g) + 1/2 O2(g) = CO2(g)  H = –283 kJ
H = –283 kJ
The heat evolved in combustion if 112 litres of water gas (mixture of equal volume of H2(g) and CO(g) is
H2(g) + 1/2 O2(g) = H2O(g)  H = –241.8 kJ
H = –241.8 kJ
CO(g) + 1/2 O2(g) = CO2(g)  H = –283 kJ
H = –283 kJ
Chemistry-General
Chemistry-
(1) For the given heat of reaction,
i) C(s) + O2(g) = CO2(g) + 97 kcal
ii) CO2(g) + C(s) = 2CO(g) – 39 kcal the heat of combustion of CO(g) is:
(1) For the given heat of reaction,
i) C(s) + O2(g) = CO2(g) + 97 kcal
ii) CO2(g) + C(s) = 2CO(g) – 39 kcal the heat of combustion of CO(g) is:
Chemistry-General
Chemistry-
A solution of CuSO4 is electrolysed using platinum inert electrodes. Due to electrolysis, the colour changes from blue to _________ and pH of the solution ________.
A solution of CuSO4 is electrolysed using platinum inert electrodes. Due to electrolysis, the colour changes from blue to _________ and pH of the solution ________.
Chemistry-General
Chemistry-
Chemistry-General
Chemistry-
An electrophilic reagent is:
An electrophilic reagent is:
Chemistry-General
Chemistry-
Which one of the following is a free radical?
Which one of the following is a free radical?
Chemistry-General
Chemistry-
In acidic medium Cr2O72– is converted to Cr3+ when it acts as an oxidising agent. The quantity of electricity required to reduce 0.05 mole of Cr2O72– would be
In acidic medium Cr2O72– is converted to Cr3+ when it acts as an oxidising agent. The quantity of electricity required to reduce 0.05 mole of Cr2O72– would be
Chemistry-General
Chemistry-
One of the constituent of german silver is
One of the constituent of german silver is
Chemistry-General
Chemistry-
Chemistry-General
Chemistry-
Chemistry-General
Chemistry-
The element which would be extracted by electrolytic reduction of its oxide dissolved in a high temperature melt is
The element which would be extracted by electrolytic reduction of its oxide dissolved in a high temperature melt is
Chemistry-General





