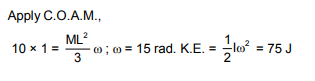Physics-
General
Easy
Question
A thin uniform straight rod of mass 2 kg and length 1 m is free to rotate about its upper end. When at rest it receives an impulsive blow of 10 Ns at its lowest point, normal to its length as shown in figure. The kinetic energy of rod just after impact is

- 75 J
- 100 J
- 200 J
- 50 J
The correct answer is: 75 J
Related Questions to study
Physics-
Find the natural frequency of oscillation of the system as shown in figure. Pulleys are massless and frictionless. Spring and string are also massless. (Take p2 = 10)

Find the natural frequency of oscillation of the system as shown in figure. Pulleys are massless and frictionless. Spring and string are also massless. (Take p2 = 10)

Physics-General
Physics-
A square loop of side ‘a’ is placed in x – y plane as shown in figure. In this region there is non-uniform time dependent magnetic field  [where t is time and c is constant] then magnitude of emf induced in loop is
[where t is time and c is constant] then magnitude of emf induced in loop is

A square loop of side ‘a’ is placed in x – y plane as shown in figure. In this region there is non-uniform time dependent magnetic field  [where t is time and c is constant] then magnitude of emf induced in loop is
[where t is time and c is constant] then magnitude of emf induced in loop is

Physics-General
Physics-
In the diagram shown, the wires P1Q1 and P2Q2 each of length 40 cm are made to slide on the rails with same speed of 5 m/s. In this region a magnetic field of 1T exists. The electric current in 9kW resistor is

In the diagram shown, the wires P1Q1 and P2Q2 each of length 40 cm are made to slide on the rails with same speed of 5 m/s. In this region a magnetic field of 1T exists. The electric current in 9kW resistor is

Physics-General
Physics-
In the circuit shown, the switch is closed at t = 0, the currents I1, I2 & I3 are

In the circuit shown, the switch is closed at t = 0, the currents I1, I2 & I3 are

Physics-General
Physics-
The instantaneous potential difference between points. A and B is :

The instantaneous potential difference between points. A and B is :

Physics-General
Physics-
Three identical large plates are fixed at separation of d from each other as shown. The area of each plate is A. Plate 1 is given charge +Q while plates 2 and 3 are neutral and are connected to each other through coil of inductances L and switch S. If resistance of all connected wires is neglected the maximum current flow through coil after closing switch is (C = e0 A/d) (neglect fringe effect)

Three identical large plates are fixed at separation of d from each other as shown. The area of each plate is A. Plate 1 is given charge +Q while plates 2 and 3 are neutral and are connected to each other through coil of inductances L and switch S. If resistance of all connected wires is neglected the maximum current flow through coil after closing switch is (C = e0 A/d) (neglect fringe effect)

Physics-General
Chemistry-
Rate of a chemical reaction can be kept constant by:
Rate of a chemical reaction can be kept constant by:
Chemistry-General
Chemistry-
Inversion of a sugar follows first order rate equation which can be followed by noting the change in rotation of the plane of polarisation of light in a polarimeter. If 1:", li and '0 are the fotations at t = 00, t = t and t = 0, then first order reaction can be written as:
Inversion of a sugar follows first order rate equation which can be followed by noting the change in rotation of the plane of polarisation of light in a polarimeter. If 1:", li and '0 are the fotations at t = 00, t = t and t = 0, then first order reaction can be written as:
Chemistry-General
Chemistry-
A substance undergoes first order decomposition. The decomposition follows two parallel first order reactions as:  The percentage distributions of Band Care:
The percentage distributions of Band Care:
A substance undergoes first order decomposition. The decomposition follows two parallel first order reactions as:  The percentage distributions of Band Care:
The percentage distributions of Band Care:
Chemistry-General
Chemistry-
Two reactions  Products and
Products and  Products, have rate constants k,4 and kB at temperature T and activation energies EA and EB respectively. If kA > kB and EA < EB and assuming that A for both the reactions is same, then:
Products, have rate constants k,4 and kB at temperature T and activation energies EA and EB respectively. If kA > kB and EA < EB and assuming that A for both the reactions is same, then:
Two reactions  Products and
Products and  Products, have rate constants k,4 and kB at temperature T and activation energies EA and EB respectively. If kA > kB and EA < EB and assuming that A for both the reactions is same, then:
Products, have rate constants k,4 and kB at temperature T and activation energies EA and EB respectively. If kA > kB and EA < EB and assuming that A for both the reactions is same, then:
Chemistry-General
Chemistry-
In the following first order competing reactions;  . The ratio of
. The ratio of  if only 50% of B will have been reacted when 94% of A has been reacted is:
if only 50% of B will have been reacted when 94% of A has been reacted is:
In the following first order competing reactions;  . The ratio of
. The ratio of  if only 50% of B will have been reacted when 94% of A has been reacted is:
if only 50% of B will have been reacted when 94% of A has been reacted is:
Chemistry-General
Chemistry-
For an endothermic reaction, where Mf represents the enthilJpy of the reaction in kJ/mol, the minimum value for the energy of activation will be:
For an endothermic reaction, where Mf represents the enthilJpy of the reaction in kJ/mol, the minimum value for the energy of activation will be:
Chemistry-General
Chemistry-
The hydrolysis of an ester was carried out separately with 0.05 NHCl and 0.05 NH2S04. Which of the following will be true?
The hydrolysis of an ester was carried out separately with 0.05 NHCl and 0.05 NH2S04. Which of the following will be true?
Chemistry-General
Chemistry-
From the following data, the activation energy for the reaction (cal/mol) is:
<img src="https://mycourses.turito.com/tokenpluginfile.php/c161933dbfaab094c54655ab71e9b8f0/1/question/questiontext/643590/1/1165511/Picture1.png" alt="" width="244" height="166"
From the following data, the activation energy for the reaction (cal/mol) is:
<img src="https://mycourses.turito.com/tokenpluginfile.php/c161933dbfaab094c54655ab71e9b8f0/1/question/questiontext/643590/1/1165511/Picture1.png" alt="" width="244" height="166"
Chemistry-General
Chemistry-
In gaseous reactions, important for the understanding of the upper atmosphere H20 and 0 react bimolecularly to form two OH radicals. Mf for this reaction is 72 kJ at 500 K and Ea is 77 kJ mol-I; then for the bimolecular recombination of two OH radicals to form H20 and 0 is:
In gaseous reactions, important for the understanding of the upper atmosphere H20 and 0 react bimolecularly to form two OH radicals. Mf for this reaction is 72 kJ at 500 K and Ea is 77 kJ mol-I; then for the bimolecular recombination of two OH radicals to form H20 and 0 is:
Chemistry-General