Chemistry-
General
Easy
Question
Calculate enthalpy of vapourization per mole of ethanol. Given ΔS=109.8JK–1mol–1andB.pt.of
ethanolis78.5°C.
- ΔSvap=
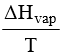
- 38.594KJmol–1
- 3.85KJmol
- Some more datais required
The correct answer is: 38.594KJmol–1
Related Questions to study
chemistry-
The enthalpy of vaporisation of per mole of ethanol (b.p.=79.5°CandS=109.8JK–1mol–1)is-
The enthalpy of vaporisation of per mole of ethanol (b.p.=79.5°CandS=109.8JK–1mol–1)is-
chemistry-General
physics
In the above question, after appropriate conditions are made, it is found that no deflection takes places in the galvanometer where the sliding jockey touches the wire at a distance of from . What is the value of the resistance X?
In the above question, after appropriate conditions are made, it is found that no deflection takes places in the galvanometer where the sliding jockey touches the wire at a distance of from . What is the value of the resistance X?
physicsGeneral
physics
A thin uniform wire AB of length 1 m, an unknown resistance X and a resistance of  are connected, by thick conducting strips as shown in figure. A battery and a galvanometer (with a sliding jockey connected to it) are also available. Connections are to be measure the unknown resistance using the principle of Wheatstone bridge. The appropriate connections are.
are connected, by thick conducting strips as shown in figure. A battery and a galvanometer (with a sliding jockey connected to it) are also available. Connections are to be measure the unknown resistance using the principle of Wheatstone bridge. The appropriate connections are.

(E is the balance point for whetstone bridge)
A thin uniform wire AB of length 1 m, an unknown resistance X and a resistance of  are connected, by thick conducting strips as shown in figure. A battery and a galvanometer (with a sliding jockey connected to it) are also available. Connections are to be measure the unknown resistance using the principle of Wheatstone bridge. The appropriate connections are.
are connected, by thick conducting strips as shown in figure. A battery and a galvanometer (with a sliding jockey connected to it) are also available. Connections are to be measure the unknown resistance using the principle of Wheatstone bridge. The appropriate connections are.

(E is the balance point for whetstone bridge)
physicsGeneral
physics
Two liquids of equal volume are thoroughly mixed. If their specific heat are  , temperatures
, temperatures  and densities
and densities  respectively. What is the final temperature of the rrixture?
respectively. What is the final temperature of the rrixture?
Two liquids of equal volume are thoroughly mixed. If their specific heat are  , temperatures
, temperatures  and densities
and densities  respectively. What is the final temperature of the rrixture?
respectively. What is the final temperature of the rrixture?
physicsGeneral
chemistry-
For which reaction from the following,ΔSwill be maximum?
For which reaction from the following,ΔSwill be maximum?
chemistry-General
chemistry-
Onheating128gofoxygengasfrom0°Cto100°C,CV and CP on an average are 5 and7calmol degree the value of ΔE and ΔH are respectively-
Onheating128gofoxygengasfrom0°Cto100°C,CV and CP on an average are 5 and7calmol degree the value of ΔE and ΔH are respectively-
chemistry-General
chemistry-
Forareaction2X(s)+2Y(s)→2C( )+D(g) The q at27°Cis–28KCal.mol–1. The qv is.......... K. Cal. mol–1
)+D(g) The q at27°Cis–28KCal.mol–1. The qv is.......... K. Cal. mol–1
Forareaction2X(s)+2Y(s)→2C( )+D(g) The q at27°Cis–28KCal.mol–1. The qv is.......... K. Cal. mol–1
)+D(g) The q at27°Cis–28KCal.mol–1. The qv is.......... K. Cal. mol–1
chemistry-General
chemistry-
For which of the following reactions ΔH is less than ΔE-
For which of the following reactions ΔH is less than ΔE-
chemistry-General
chemistry-
For which change ΔH≠ΔE–
For which change ΔH≠ΔE–
chemistry-General
chemistry-
Which is true for the combustion of sucrose (C12H22O11)at25°C-
Which is true for the combustion of sucrose (C12H22O11)at25°C-
chemistry-General
physics
The temperature of equal mases of three different liquids X,Y, zare  and
and  respectively The temperature when X and Y aremixed is
respectively The temperature when X and Y aremixed is  and when Y and Z are mixed is
and when Y and Z are mixed is  what is the temperature when X and Z are mixed?
what is the temperature when X and Z are mixed?
The temperature of equal mases of three different liquids X,Y, zare  and
and  respectively The temperature when X and Y aremixed is
respectively The temperature when X and Y aremixed is  and when Y and Z are mixed is
and when Y and Z are mixed is  what is the temperature when X and Z are mixed?
what is the temperature when X and Z are mixed?
physicsGeneral
physics
For which value of the temperature will the values of Fahrenheit scale and Kelvin scale be equal?
For which value of the temperature will the values of Fahrenheit scale and Kelvin scale be equal?
physicsGeneral
physics
On a hot day at Ahmedabad a trucker loaded 37,000 L of diesel fuel. He delivered the diesel at Srinagar (Kashmir) Where the temperature was lower then that of Ahmedabad by 23K . How many liters did he deliver ?For diesel y=3a= (Neglect the thermal expansion/ Contraction of steel tank of the trunk)
(Neglect the thermal expansion/ Contraction of steel tank of the trunk)
On a hot day at Ahmedabad a trucker loaded 37,000 L of diesel fuel. He delivered the diesel at Srinagar (Kashmir) Where the temperature was lower then that of Ahmedabad by 23K . How many liters did he deliver ?For diesel y=3a= (Neglect the thermal expansion/ Contraction of steel tank of the trunk)
(Neglect the thermal expansion/ Contraction of steel tank of the trunk)
physicsGeneral
chemistry-
For the system S(s)+O2(g)→SO2(g)-
For the system S(s)+O2(g)→SO2(g)-
chemistry-General
chemistry-
The difference in ΔH and ΔE for the combustion of methaneat25°Cwouldbe-
The difference in ΔH and ΔE for the combustion of methaneat25°Cwouldbe-
chemistry-General



