Chemistry-
General
Easy
Question
Hydrogen can be obtained from water by
- Reaction with metal oxides
- Reaction with non-metal oxides
- Reaction with metals
- Reaction with metal hydrides
The correct answer is: Reaction with metal hydrides
Related Questions to study
chemistry-
Hydrogen peroxide is
Hydrogen peroxide is
chemistry-General
chemistry-
When zeolite, which is hydrated sodium aluminium silicate, is treated with hard water the sodium ions are exchanged with
When zeolite, which is hydrated sodium aluminium silicate, is treated with hard water the sodium ions are exchanged with
chemistry-General
physics
The velocity with which a projectile must be fired so that it escapes earth's gravitational does not depend on ……
The velocity with which a projectile must be fired so that it escapes earth's gravitational does not depend on ……
physicsGeneral
chemistry-
The structure of  is
is
The structure of  is
is
chemistry-General
physics
Escape velocity on the surface of earth is 11.2kms-1 Escape velocity from a planet whose masses the same as that of earth and radius 1/4 that of earth is.....kms-1
Escape velocity on the surface of earth is 11.2kms-1 Escape velocity from a planet whose masses the same as that of earth and radius 1/4 that of earth is.....kms-1
physicsGeneral
chemistry-
Correct configuration of the following molecule is : 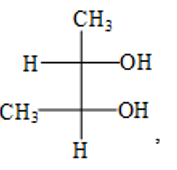 ,
,
Correct configuration of the following molecule is :  ,
,
chemistry-General
physics
3 Particle each of mass are kept at vertices of an equilateral triangle of side L. The gravitatioml field at center due to these particles is……
3 Particle each of mass are kept at vertices of an equilateral triangle of side L. The gravitatioml field at center due to these particles is……
physicsGeneral
physics
Given mass of the moon is  of the mass of the earth and corresponding radius is
of the mass of the earth and corresponding radius is  of the earth, If escape velocity on the earth surface is 11.2kms-1 the value of same on the surface of moon is.....kms-1
of the earth, If escape velocity on the earth surface is 11.2kms-1 the value of same on the surface of moon is.....kms-1
Given mass of the moon is  of the mass of the earth and corresponding radius is
of the mass of the earth and corresponding radius is  of the earth, If escape velocity on the earth surface is 11.2kms-1 the value of same on the surface of moon is.....kms-1
of the earth, If escape velocity on the earth surface is 11.2kms-1 the value of same on the surface of moon is.....kms-1
physicsGeneral
physics
The escape velocity of a body on the surface of the earth is 11.2km/sec. If the mass of the earth is increases to twice its present value and the radius of the earth becomes half, the escape velocity becomes...kms-1
The escape velocity of a body on the surface of the earth is 11.2km/sec. If the mass of the earth is increases to twice its present value and the radius of the earth becomes half, the escape velocity becomes...kms-1
physicsGeneral
physics
There are two planets, the ratio of radius of two planets is k but the acceleration due to gravity of both planets are g what will be the ratio of their escape velocity.
There are two planets, the ratio of radius of two planets is k but the acceleration due to gravity of both planets are g what will be the ratio of their escape velocity.
physicsGeneral
physics
The escape velocity of a planet having mass 6 times and radius 2 times as that of earth is.........
The escape velocity of a planet having mass 6 times and radius 2 times as that of earth is.........
physicsGeneral
chemistry-
Correct statements from the graph

I) Above 1073K,  for the formation of Fe2O3 is less negative than
for the formation of Fe2O3 is less negative than  for the formation of CO from carbon
for the formation of CO from carbon
II) Above 1073K, Carbon can reduce Fe2O3
III) Below 1073K, CO can reduce Fe2O3
IV) In blast furnace, reduction of Fe2O3 occurs in different temperature ranges with below 1073K by CO (or) above 1073K by carbon
Correct statements from the graph

I) Above 1073K,  for the formation of Fe2O3 is less negative than
for the formation of Fe2O3 is less negative than  for the formation of CO from carbon
for the formation of CO from carbon
II) Above 1073K, Carbon can reduce Fe2O3
III) Below 1073K, CO can reduce Fe2O3
IV) In blast furnace, reduction of Fe2O3 occurs in different temperature ranges with below 1073K by CO (or) above 1073K by carbon
chemistry-General
chemistry-
Correct statement(s) regarding the graph

Correct statement(s) regarding the graph

chemistry-General
chemistry-
From the graph, which is the best reducing agent to reduce Cu2 O at high temperature

From the graph, which is the best reducing agent to reduce Cu2 O at high temperature

chemistry-General
chemistry-
Correct statement(s) regarding the graph

I) Above 983K, Carbon can reduce any metal oxide at high temperature and itself oxidised to CO
II) In the first reaction (Formation of CO2 from ‘C’)  =0 &
=0 &  remains nearly same, i.e it is independent of temperature
remains nearly same, i.e it is independent of temperature
III) In the second reaction, (formation of CO) , there is increase in entropy &  =+ve&
=+ve& becomes more –ve with increase in temperature
becomes more –ve with increase in temperature
IV) In third reaction (formation of CO2 from CO), there is a decrease in entropy  =-ve &
=-ve &  becomes less –ve with increase in temperature
becomes less –ve with increase in temperature
Correct statement(s) regarding the graph

I) Above 983K, Carbon can reduce any metal oxide at high temperature and itself oxidised to CO
II) In the first reaction (Formation of CO2 from ‘C’)  =0 &
=0 &  remains nearly same, i.e it is independent of temperature
remains nearly same, i.e it is independent of temperature
III) In the second reaction, (formation of CO) , there is increase in entropy &  =+ve&
=+ve& becomes more –ve with increase in temperature
becomes more –ve with increase in temperature
IV) In third reaction (formation of CO2 from CO), there is a decrease in entropy  =-ve &
=-ve &  becomes less –ve with increase in temperature
becomes less –ve with increase in temperature
chemistry-General



