Physics
General
Easy
Question
Consider the potentiometer circuit shown. The potentiometer wire is 600 cm long and having restance 15r At what distance from the point A should the jockey touch the wire to get zero deflection in the galvanometer?

- 450 cm
- 320 cm
- 420 cm
- 360 cm
The correct answer is: 320 cm
Related Questions to study
physics-
The strain-stress curves of three wires of different materials are shown in the figure.  and
and are the elastic limits of the wires. The figure shows that
are the elastic limits of the wires. The figure shows that
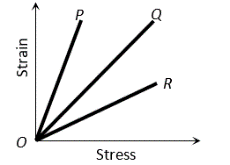
The strain-stress curves of three wires of different materials are shown in the figure.  and
and are the elastic limits of the wires. The figure shows that
are the elastic limits of the wires. The figure shows that

physics-General
chemistry-
Which of the following equations corresponds to theenthalpyofcombustionat298K:-
Which of the following equations corresponds to theenthalpyofcombustionat298K:-
chemistry-General
chemistry-
IfC6H12O6(s)+9O2(g)→6CO2(g)+6H2O(g); ΔH=–680Kcal The weight of CO2(g) produced when170Kcal of heat is evolved in the combustion
IfC6H12O6(s)+9O2(g)→6CO2(g)+6H2O(g); ΔH=–680Kcal The weight of CO2(g) produced when170Kcal of heat is evolved in the combustion
chemistry-General
chemistry-
TheenthalpyofformationforC2H4(g),CO2(g) and H2O( ) at 25°C and 1atm. pressure are 52,–394 and –286KJmol–1 respectively. The enthalpy of combustion of C2H4 will be-
) at 25°C and 1atm. pressure are 52,–394 and –286KJmol–1 respectively. The enthalpy of combustion of C2H4 will be-
TheenthalpyofformationforC2H4(g),CO2(g) and H2O( ) at 25°C and 1atm. pressure are 52,–394 and –286KJmol–1 respectively. The enthalpy of combustion of C2H4 will be-
) at 25°C and 1atm. pressure are 52,–394 and –286KJmol–1 respectively. The enthalpy of combustion of C2H4 will be-
chemistry-General
chemistry-
Accordingtoequation, CH( )+15/2O(g)→6CO(g)+3HO(
)+15/2O(g)→6CO(g)+3HO( ) ΔH= –3264.4KJ mol the energy evolved when7.8g benzene is burn tin air will be-
) ΔH= –3264.4KJ mol the energy evolved when7.8g benzene is burn tin air will be-
Accordingtoequation, CH( )+15/2O(g)→6CO(g)+3HO(
)+15/2O(g)→6CO(g)+3HO( ) ΔH= –3264.4KJ mol the energy evolved when7.8g benzene is burn tin air will be-
) ΔH= –3264.4KJ mol the energy evolved when7.8g benzene is burn tin air will be-
chemistry-General
chemistry-
M is a metal that forms an oxide  When a sample of metal M reacts with one mole of oxygen what will be the ΔH in that case-
When a sample of metal M reacts with one mole of oxygen what will be the ΔH in that case-
M is a metal that forms an oxide  When a sample of metal M reacts with one mole of oxygen what will be the ΔH in that case-
When a sample of metal M reacts with one mole of oxygen what will be the ΔH in that case-
chemistry-General
physics
Circuit for the measurement of resistance by potentiometer is shown. The galvanometer is first connected at point-A and zero-deflection is observed at length PJ=10 cm. In the second case, it is connected at a point C and zero deflection is observed at a length 30 cm from . Then what is the unknown resistance X?

Circuit for the measurement of resistance by potentiometer is shown. The galvanometer is first connected at point-A and zero-deflection is observed at length PJ=10 cm. In the second case, it is connected at a point C and zero deflection is observed at a length 30 cm from . Then what is the unknown resistance X?

physicsGeneral
physics
A potentiometer is preferred over a voltmeter to measure the emf of a cell because....
A potentiometer is preferred over a voltmeter to measure the emf of a cell because....
physicsGeneral
physics
A 10 m wire potentiometer is connected to an accumulator of steady voltage. A 7.8 m length of it balances the emf of a cell on 'open-circuit'. When cell delivers current through a conductor of resistance  Ω it is balanced against 7.0 cm of the same poleutomelic. What is the internal resistance of the cell?
Ω it is balanced against 7.0 cm of the same poleutomelic. What is the internal resistance of the cell?
A 10 m wire potentiometer is connected to an accumulator of steady voltage. A 7.8 m length of it balances the emf of a cell on 'open-circuit'. When cell delivers current through a conductor of resistance  Ω it is balanced against 7.0 cm of the same poleutomelic. What is the internal resistance of the cell?
Ω it is balanced against 7.0 cm of the same poleutomelic. What is the internal resistance of the cell?
physicsGeneral
chemistry-
The structure of the following polymer is :

The structure of the following polymer is :

chemistry-General
physics
The reciprocal of resistivity is called…….
The reciprocal of resistivity is called…….
physicsGeneral
physics
If a copper wire is stretched to make it n=0.1% longer, then what is the percentage change in its resistance?
If a copper wire is stretched to make it n=0.1% longer, then what is the percentage change in its resistance?
physicsGeneral
chemistry-
The standard molar heat of formation of ethane, CO2,andwater( ) are respectively–21.1,–94.1and –68.3Kcal.Thestandardmolarheatofcombustion of ethane will be-
) are respectively–21.1,–94.1and –68.3Kcal.Thestandardmolarheatofcombustion of ethane will be-
The standard molar heat of formation of ethane, CO2,andwater( ) are respectively–21.1,–94.1and –68.3Kcal.Thestandardmolarheatofcombustion of ethane will be-
) are respectively–21.1,–94.1and –68.3Kcal.Thestandardmolarheatofcombustion of ethane will be-
chemistry-General
chemistry-
Given that standard heat enthalph of CH4,C2H4 andC3H8are–17.9,12.5,–24.8Kcal/mol. The ΔH for CH4+C2H4→C3H8 is-
Given that standard heat enthalph of CH4,C2H4 andC3H8are–17.9,12.5,–24.8Kcal/mol. The ΔH for CH4+C2H4→C3H8 is-
chemistry-General
chemistry-
The enthalpy of formation of ammonia is–46.0KJ mol–1.The enthalpy change for the reaction 2NH3(g)→N2(g)+3H2(g) is-
The enthalpy of formation of ammonia is–46.0KJ mol–1.The enthalpy change for the reaction 2NH3(g)→N2(g)+3H2(g) is-
chemistry-General





