Chemistry-
General
Easy
Question
For a valid Bohr orbit, its cicumfrence should be -
- nλ
- (n-1)λ
- >nλ
- <λ
The correct answer is: nλ
Related Questions to study
chemistry-
Arrange the following particles in increasing order of values of e/m ratio: Electron (e), proton (p), neutron (n) and α-particle (α) -
Arrange the following particles in increasing order of values of e/m ratio: Electron (e), proton (p), neutron (n) and α-particle (α) -
chemistry-General
Maths-
In 
In 
Maths-General
physics-
An antenna behaves as resonant circuit only when its length is
An antenna behaves as resonant circuit only when its length is
physics-General
Maths-
If 
If 
Maths-General
physics-
In the adjoining diagram, a wavefront AB, moving in air is incident on a plane glass surface XY. Its position CD after refraction through a glass slab is shown also along with the normals drawn at A and D. The refractive index of glass with respect to air ( ) will be equal to
) will be equal to

In the adjoining diagram, a wavefront AB, moving in air is incident on a plane glass surface XY. Its position CD after refraction through a glass slab is shown also along with the normals drawn at A and D. The refractive index of glass with respect to air ( ) will be equal to
) will be equal to

physics-General
chemistry-
Which statement is not correct for n=5,m=2:
Which statement is not correct for n=5,m=2:
chemistry-General
chemistry-
In P-atom find out the no. of paired electrons for l=1 and m =0-
In P-atom find out the no. of paired electrons for l=1 and m =0-
chemistry-General
chemistry-
For H- atom, the energy required for the removal electron from various sub-shells is given at under:
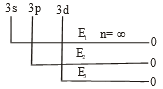 The order of the energies would be:
The order of the energies would be:
For H- atom, the energy required for the removal electron from various sub-shells is given at under:
 The order of the energies would be:
The order of the energies would be:
chemistry-General
physics-
Immiscible transparent liquids A, B, C, D and E are placed in a rectangular container of glass with the liquids making layers according to their densities. The refractive index of the liquids are shown in the adjoining diagram. The container is illuminated from the side and a small piece of glass having refractive index 1.61 is gently dropped into the liquid layer. The glass piece as it descends downwards will not be visible in

Immiscible transparent liquids A, B, C, D and E are placed in a rectangular container of glass with the liquids making layers according to their densities. The refractive index of the liquids are shown in the adjoining diagram. The container is illuminated from the side and a small piece of glass having refractive index 1.61 is gently dropped into the liquid layer. The glass piece as it descends downwards will not be visible in

physics-General
Maths-
Values of  is :
is :
Values of  is :
is :
Maths-General
physics-
A rectangular tank of depth 8 meter is full of water ( ), the bottom is seen at the depth
), the bottom is seen at the depth
A rectangular tank of depth 8 meter is full of water ( ), the bottom is seen at the depth
), the bottom is seen at the depth
physics-General
chemistry-
A photon of energe12.75ev is completely absorbed by a hydrogen atom initially in ground sate. The principle quantum number of the excited state is:
A photon of energe12.75ev is completely absorbed by a hydrogen atom initially in ground sate. The principle quantum number of the excited state is:
chemistry-General
chemistry-
A certain electronic transition from an excited statetothegroundstateoftheH2 atom in one or more steps gives rise to four lines in the ultra violet region of the spectrum, how many lines does this transition produce in the infrared region of the spectrum-
A certain electronic transition from an excited statetothegroundstateoftheH2 atom in one or more steps gives rise to four lines in the ultra violet region of the spectrum, how many lines does this transition produce in the infrared region of the spectrum-
chemistry-General
chemistry-
Find out ratio of following for photon (νmax.) Lyman: (νmax) Brackett-
Find out ratio of following for photon (νmax.) Lyman: (νmax) Brackett-
chemistry-General
chemistry-
The potential energies of first, second and third Bohr’s orbits of He+ cation are E1,E2 and E3. The correct sequence of these energies is-
The potential energies of first, second and third Bohr’s orbits of He+ cation are E1,E2 and E3. The correct sequence of these energies is-
chemistry-General





