Question
- 1
- 0
- -1

The correct answer is: 0
Related Questions to study
The work per unit volume to stretch the length by 1% of a wire with cross - section area 1 mm2 will be.....
The work per unit volume to stretch the length by 1% of a wire with cross - section area 1 mm2 will be.....
We can only apply the L’Hospital’s rule if the direct substitution returns an indeterminate form, that means
We can only apply the L’Hospital’s rule if the direct substitution returns an indeterminate form, that means
We can only apply the L’Hospital’s rule if the direct substitution returns an indeterminate form, that means 0 over 0 or
We can only apply the L’Hospital’s rule if the direct substitution returns an indeterminate form, that means 0 over 0 or
We can only apply the L’Hospital’s rule if the direct substitution returns an indeterminate form, that means 0 over 0 or
We can only apply the L’Hospital’s rule if the direct substitution returns an indeterminate form, that means 0 over 0 or
Young's modulus of the material of a wire is Y. On pulling the wire by a force F the increase in its length is x, what is the potential energy of the stretched wire?
Young's modulus of the material of a wire is Y. On pulling the wire by a force F the increase in its length is x, what is the potential energy of the stretched wire?
We can only apply the L’Hospital’s rule if the direct substitution returns an indeterminate form, that means 0 over 0 or
We can only apply the L’Hospital’s rule if the direct substitution returns an indeterminate form, that means 0 over 0 or
The figure shows P–V diagram of a thermodynamic cycle If  are the respective temperature at A, B, C and D Then, choose the correct statement if
are the respective temperature at A, B, C and D Then, choose the correct statement if 

The figure shows P–V diagram of a thermodynamic cycle If  are the respective temperature at A, B, C and D Then, choose the correct statement if
are the respective temperature at A, B, C and D Then, choose the correct statement if 

The figure shows P–V diagram of a thermodynamic cycle The work done by the cycle is

The figure shows P–V diagram of a thermodynamic cycle The work done by the cycle is

A monoatomic ideal gas sample is given heat Q One fourth of this heat is used as work done by the gas and rest is used for increasing its internal energy An ideal gas under goes a thermodynamic cycle as shown in fig Which of the following graphs represents the same cycle?
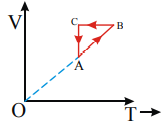
A) 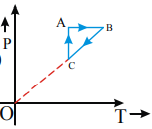
B) 
C) 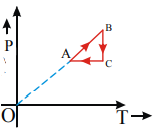
D) 
A monoatomic ideal gas sample is given heat Q One fourth of this heat is used as work done by the gas and rest is used for increasing its internal energy An ideal gas under goes a thermodynamic cycle as shown in fig Which of the following graphs represents the same cycle?

A) 
B) 
C) 
D) 
A monoatomic ideal gas sample is given heat Q One fourth of this heat is used as work done by the gas and rest is used for increasing its internal energy The P V diagram for the process is
A monoatomic ideal gas sample is given heat Q One fourth of this heat is used as work done by the gas and rest is used for increasing its internal energy The P V diagram for the process is
The V–T diagram of an ideal gas for the process  (straight lines) is as shown in the figure In the process
(straight lines) is as shown in the figure In the process 
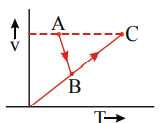
A) pressure is always increasing
B) for some interval pressure decreases but finally pressure is more than initial pressure
C) pressure first increases then remains constant
D) graph AB is unpredictable about pressure
The V–T diagram of an ideal gas for the process  (straight lines) is as shown in the figure In the process
(straight lines) is as shown in the figure In the process 

A) pressure is always increasing
B) for some interval pressure decreases but finally pressure is more than initial pressure
C) pressure first increases then remains constant
D) graph AB is unpredictable about pressure
Statement-1: Two vessels A and B are connected to each other by a stopcock .Vessel A contains a gas at 300K and 1 atmosphere pressure and vessel B is evacuated The two vessels are thermally insulated from the surroundings If the stopcock is suddenly opened, the expanding gas does no work
Statement-2: Since D Q = 0 and as the gas expands freely so DW = 0 and from the first law of thermodynamics it follows that DU is also zero for the above process
Statement-1: Two vessels A and B are connected to each other by a stopcock .Vessel A contains a gas at 300K and 1 atmosphere pressure and vessel B is evacuated The two vessels are thermally insulated from the surroundings If the stopcock is suddenly opened, the expanding gas does no work
Statement-2: Since D Q = 0 and as the gas expands freely so DW = 0 and from the first law of thermodynamics it follows that DU is also zero for the above process
We can only apply the L’Hospital’s rule if the direct substitution returns an indeterminate form, that means 0 over 0 or
We can only apply the L’Hospital’s rule if the direct substitution returns an indeterminate form, that means 0 over 0 or



