Maths-
General
Easy
Question
In the following figure, a pair of adjacent vertices is

- X, Y
- A, F
- X, L
- P, F
The correct answer is: A, F
Pair of adjacent vertices are A,F
Related Questions to study
chemistry-
Kinetic theory of gases is a generalization offered by Maxwell, Boltzmann, Clausius, etc., to explain the behavior of ideal gases. This theory assumes that ideal gas molecules neither attract nor repel each other. Average kinetic energy of gas molecules is directly proportional to the absolute temperature. A gas equation called kinetic gas equation was derived on the basis of kinetic theory
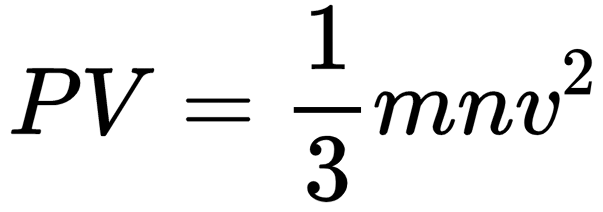

Pick up the correct statement/statements: 1. gas A will tend to lie at the bottom. 2. the number of atoms of various gases A, Band Care same. 3. the gases will diffuse to form homogeneous mixture, 4. average kinetic energy of each gas is same,
Kinetic theory of gases is a generalization offered by Maxwell, Boltzmann, Clausius, etc., to explain the behavior of ideal gases. This theory assumes that ideal gas molecules neither attract nor repel each other. Average kinetic energy of gas molecules is directly proportional to the absolute temperature. A gas equation called kinetic gas equation was derived on the basis of kinetic theory
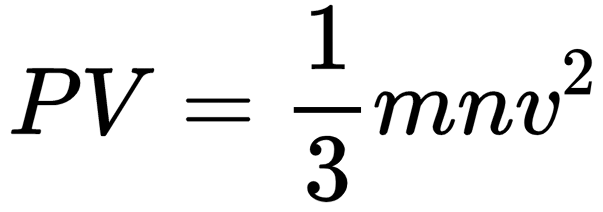

Pick up the correct statement/statements: 1. gas A will tend to lie at the bottom. 2. the number of atoms of various gases A, Band Care same. 3. the gases will diffuse to form homogeneous mixture, 4. average kinetic energy of each gas is same,
chemistry-General
chemistry-
The essential conditions for liquefaction of gases were discovered by Andrews in 1869 as a result of his study of pressure-volume-temperature relationship for  , It was found that above a certain temperature, it was impossible to liquefy a gas whatever the pressure was applied. The temperature below which the gas can be liquefied by the application of pressure alone is called critical temperature
, It was found that above a certain temperature, it was impossible to liquefy a gas whatever the pressure was applied. The temperature below which the gas can be liquefied by the application of pressure alone is called critical temperature  . The pressure required to liquefy a gas at this temperature is called the critical pressure
. The pressure required to liquefy a gas at this temperature is called the critical pressure  The volume occupied by one m91e of the substance at the critical temperature and pressure is called critical volume. Critical constants are related with van der Waals' constant as follows:
The volume occupied by one m91e of the substance at the critical temperature and pressure is called critical volume. Critical constants are related with van der Waals' constant as follows:


Which of the above gases cannot be liquefied at 100 K and 50 - atm?
The essential conditions for liquefaction of gases were discovered by Andrews in 1869 as a result of his study of pressure-volume-temperature relationship for  , It was found that above a certain temperature, it was impossible to liquefy a gas whatever the pressure was applied. The temperature below which the gas can be liquefied by the application of pressure alone is called critical temperature
, It was found that above a certain temperature, it was impossible to liquefy a gas whatever the pressure was applied. The temperature below which the gas can be liquefied by the application of pressure alone is called critical temperature  . The pressure required to liquefy a gas at this temperature is called the critical pressure
. The pressure required to liquefy a gas at this temperature is called the critical pressure  The volume occupied by one m91e of the substance at the critical temperature and pressure is called critical volume. Critical constants are related with van der Waals' constant as follows:
The volume occupied by one m91e of the substance at the critical temperature and pressure is called critical volume. Critical constants are related with van der Waals' constant as follows:


Which of the above gases cannot be liquefied at 100 K and 50 - atm?
chemistry-General
chemistry-
The packing efficiency of the two-dimensional square unit cell shown below is:

The packing efficiency of the two-dimensional square unit cell shown below is:

chemistry-General
chemistry-
The volume-temperature 'graphs of a given mass of an ideal gas at constant pressures are shown below. What is the correct order of pressures?

The volume-temperature 'graphs of a given mass of an ideal gas at constant pressures are shown below. What is the correct order of pressures?

chemistry-General
physics-
For measuring depth of a beaker using verniercallipers observed readings are given as If zero error is - 0.03cm, then mean corrected depth is

For measuring depth of a beaker using verniercallipers observed readings are given as If zero error is - 0.03cm, then mean corrected depth is

physics-General
physics-
The n th division of main scale coincides with  division of vernierscale Given+n one main scale division is equal to 'a' units The least count of vernier is
division of vernierscale Given+n one main scale division is equal to 'a' units The least count of vernier is
The n th division of main scale coincides with  division of vernierscale Given+n one main scale division is equal to 'a' units The least count of vernier is
division of vernierscale Given+n one main scale division is equal to 'a' units The least count of vernier is
physics-General
physics-
The nuclear charge (Ze) is non-uniformly distributed within a nucleus of radius R The (charge per unit volume)  charge density is dependent only on the radial distance r from the centre of the nucleus, as shown The electric field is only along radial direction
charge density is dependent only on the radial distance r from the centre of the nucleus, as shown The electric field is only along radial direction
For a = 0, the value of d(maximum value of  as shown in the figure) is :
as shown in the figure) is :

The nuclear charge (Ze) is non-uniformly distributed within a nucleus of radius R The (charge per unit volume)  charge density is dependent only on the radial distance r from the centre of the nucleus, as shown The electric field is only along radial direction
charge density is dependent only on the radial distance r from the centre of the nucleus, as shown The electric field is only along radial direction
For a = 0, the value of d(maximum value of  as shown in the figure) is :
as shown in the figure) is :

physics-General
physics-
The nuclear charge (Ze) is non-uniformly distributed within a nucleus of radius R The (charge per unit volume)  charge density is dependent only on the radial distance r from the centre of the nucleus, as shown The electric field is only along radial direction
charge density is dependent only on the radial distance r from the centre of the nucleus, as shown The electric field is only along radial direction

The electric field at r = R is :
The nuclear charge (Ze) is non-uniformly distributed within a nucleus of radius R The (charge per unit volume)  charge density is dependent only on the radial distance r from the centre of the nucleus, as shown The electric field is only along radial direction
charge density is dependent only on the radial distance r from the centre of the nucleus, as shown The electric field is only along radial direction

The electric field at r = R is :
physics-General
physics-
Consider radioactive decay of A to B which , further decays either to X or Y, and are decay constants for A to B decay, B to X decay and B to Y decay respectively At t =0, the number of nuclei of A,B,X and Y are
are decay constants for A to B decay, B to X decay and B to Y decay respectively At t =0, the number of nuclei of A,B,X and Y are  ,
,  zero and zero respectively
zero and zero respectively  are the number of nuclei of A,B,X and Y are at any instant t.
are the number of nuclei of A,B,X and Y are at any instant t.

At  , which of following is incorrect?
, which of following is incorrect?
Consider radioactive decay of A to B which , further decays either to X or Y, and are decay constants for A to B decay, B to X decay and B to Y decay respectively At t =0, the number of nuclei of A,B,X and Y are
are decay constants for A to B decay, B to X decay and B to Y decay respectively At t =0, the number of nuclei of A,B,X and Y are  ,
,  zero and zero respectively
zero and zero respectively  are the number of nuclei of A,B,X and Y are at any instant t.
are the number of nuclei of A,B,X and Y are at any instant t.

At  , which of following is incorrect?
, which of following is incorrect?
physics-General
physics-
Consider radioactive decay of A to B which , further decays either to X or Y, and are decay constants for A to B decay, B to X decay and B to Y decay respectively At t =0, the number of nuclei of A,B,X and Y are
are decay constants for A to B decay, B to X decay and B to Y decay respectively At t =0, the number of nuclei of A,B,X and Y are  ,
,  zero and zero respectively
zero and zero respectively  are the number of nuclei of A,B,X and Y are at any instant t.
are the number of nuclei of A,B,X and Y are at any instant t.

The number of nuclei of B will first increase and then after a maximum value, it decreases for
Consider radioactive decay of A to B which , further decays either to X or Y, and are decay constants for A to B decay, B to X decay and B to Y decay respectively At t =0, the number of nuclei of A,B,X and Y are
are decay constants for A to B decay, B to X decay and B to Y decay respectively At t =0, the number of nuclei of A,B,X and Y are  ,
,  zero and zero respectively
zero and zero respectively  are the number of nuclei of A,B,X and Y are at any instant t.
are the number of nuclei of A,B,X and Y are at any instant t.

The number of nuclei of B will first increase and then after a maximum value, it decreases for
physics-General
physics-
Consider radioactive decay of A to B which , further decays either to X or Y, and are decay constants for A to B decay, B to X decay and B to Y decay respectively At t =0, the number of nuclei of A,B,X and Y are
are decay constants for A to B decay, B to X decay and B to Y decay respectively At t =0, the number of nuclei of A,B,X and Y are  ,
,  zero and zero respectively
zero and zero respectively  are the number of nuclei of A,B,X and Y are at any instant t.
are the number of nuclei of A,B,X and Y are at any instant t.

The net rate of accumulation of B at any instant is
Consider radioactive decay of A to B which , further decays either to X or Y, and are decay constants for A to B decay, B to X decay and B to Y decay respectively At t =0, the number of nuclei of A,B,X and Y are
are decay constants for A to B decay, B to X decay and B to Y decay respectively At t =0, the number of nuclei of A,B,X and Y are  ,
,  zero and zero respectively
zero and zero respectively  are the number of nuclei of A,B,X and Y are at any instant t.
are the number of nuclei of A,B,X and Y are at any instant t.

The net rate of accumulation of B at any instant is
physics-General
physics-
The mass of nucleus  is less than the sum of the mases of (A - Z) number of neutrons and Z number of protons in the nucleus The energy equivalent to the corresponding mass difference is known as the binding energy of the nucleus A heavy nucleus of mass M can break into two light nuclei of mass m1 and m2 M only if
is less than the sum of the mases of (A - Z) number of neutrons and Z number of protons in the nucleus The energy equivalent to the corresponding mass difference is known as the binding energy of the nucleus A heavy nucleus of mass M can break into two light nuclei of mass m1 and m2 M only if  Also two light nuclei of mass m3 and m4 can undergo complete fusion and form a heavy nucleus of mass M “only if
Also two light nuclei of mass m3 and m4 can undergo complete fusion and form a heavy nucleus of mass M “only if  The masses of some netural atoms are given in the table below.
The masses of some netural atoms are given in the table below.

The kinetic energy (in keV) of the alpha particle, when the nucleus at rest undergo alpha decay, is
The mass of nucleus  is less than the sum of the mases of (A - Z) number of neutrons and Z number of protons in the nucleus The energy equivalent to the corresponding mass difference is known as the binding energy of the nucleus A heavy nucleus of mass M can break into two light nuclei of mass m1 and m2 M only if
is less than the sum of the mases of (A - Z) number of neutrons and Z number of protons in the nucleus The energy equivalent to the corresponding mass difference is known as the binding energy of the nucleus A heavy nucleus of mass M can break into two light nuclei of mass m1 and m2 M only if  Also two light nuclei of mass m3 and m4 can undergo complete fusion and form a heavy nucleus of mass M “only if
Also two light nuclei of mass m3 and m4 can undergo complete fusion and form a heavy nucleus of mass M “only if  The masses of some netural atoms are given in the table below.
The masses of some netural atoms are given in the table below.

The kinetic energy (in keV) of the alpha particle, when the nucleus at rest undergo alpha decay, is
physics-General
physics-
The mass of nucleus  is less than the sum of the mases of (A - Z) number of neutrons and Z number of protons in the nucleus The energy equivalent to the corresponding mass difference is known as the binding energy of the nucleus A heavy nucleus of mass M can break into two light nuclei of mass m1 and m2 M only if
is less than the sum of the mases of (A - Z) number of neutrons and Z number of protons in the nucleus The energy equivalent to the corresponding mass difference is known as the binding energy of the nucleus A heavy nucleus of mass M can break into two light nuclei of mass m1 and m2 M only if  Also two light nuclei of mass m3 and m4 can undergo complete fusion and form a heavy nucleus of mass M “only if
Also two light nuclei of mass m3 and m4 can undergo complete fusion and form a heavy nucleus of mass M “only if  The masses of some netrural atoms are given in the table below.
The masses of some netrural atoms are given in the table below.

The correct statement is
The mass of nucleus  is less than the sum of the mases of (A - Z) number of neutrons and Z number of protons in the nucleus The energy equivalent to the corresponding mass difference is known as the binding energy of the nucleus A heavy nucleus of mass M can break into two light nuclei of mass m1 and m2 M only if
is less than the sum of the mases of (A - Z) number of neutrons and Z number of protons in the nucleus The energy equivalent to the corresponding mass difference is known as the binding energy of the nucleus A heavy nucleus of mass M can break into two light nuclei of mass m1 and m2 M only if  Also two light nuclei of mass m3 and m4 can undergo complete fusion and form a heavy nucleus of mass M “only if
Also two light nuclei of mass m3 and m4 can undergo complete fusion and form a heavy nucleus of mass M “only if  The masses of some netrural atoms are given in the table below.
The masses of some netrural atoms are given in the table below.

The correct statement is
physics-General
physics-
From the figure describing photoelectric effect we may infer correctly that

From the figure describing photoelectric effect we may infer correctly that

physics-General
physics-
Figure represents a graph of kinetic energy of most energetic photoelectrons, Kmax (in eV), and frequency (v) for a metal used as cathode in photoelectric experiment. The threshold frequency of light for the photoelectric emission from the metal is

Figure represents a graph of kinetic energy of most energetic photoelectrons, Kmax (in eV), and frequency (v) for a metal used as cathode in photoelectric experiment. The threshold frequency of light for the photoelectric emission from the metal is

physics-General



