Maths-
General
Easy
Question
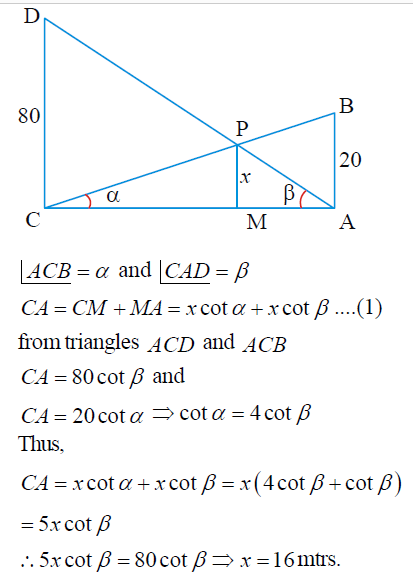
Two vertical poles 20m and 80m heigh stand apart on a horizontal plane The height of the point of intersection of the lines joining the top of each pole to the foot of the other is
The correct answer is: 
Related Questions to study
chemistry-
5 mole of an ideal gas expand isothermally and irreversibly from a pressure of 10atm to 1atm against a constant external pressure of 1 atm.  at 300 K is :
at 300 K is :
5 mole of an ideal gas expand isothermally and irreversibly from a pressure of 10atm to 1atm against a constant external pressure of 1 atm.  at 300 K is :
at 300 K is :
chemistry-General
chemistry-
In the conversion of lime stone to lime,  the values of
the values of  and
and  are
are  and
and  respectively at
respectively at  and 1 bar. Assuming,
and 1 bar. Assuming, and
and  do not change with temperature; temperature above which conversion of lime stone to lime will be spontaneous is
do not change with temperature; temperature above which conversion of lime stone to lime will be spontaneous is
In the conversion of lime stone to lime,  the values of
the values of  and
and  are
are  and
and  respectively at
respectively at  and 1 bar. Assuming,
and 1 bar. Assuming, and
and  do not change with temperature; temperature above which conversion of lime stone to lime will be spontaneous is
do not change with temperature; temperature above which conversion of lime stone to lime will be spontaneous is
chemistry-General
chemistry-
Which of the following expressions is correct?
Which of the following expressions is correct?
chemistry-General
chemistry-
Which of the following is true for spontaneous process?
Which of the following is true for spontaneous process?
chemistry-General
chemistry-
ΔG for the reaction  is -4.606 kcal. The value of equilibrium constant of the reaction at 227°C is
is -4.606 kcal. The value of equilibrium constant of the reaction at 227°C is
ΔG for the reaction  is -4.606 kcal. The value of equilibrium constant of the reaction at 227°C is
is -4.606 kcal. The value of equilibrium constant of the reaction at 227°C is
chemistry-General
chemistry-
Driving force of a reaction is the
Driving force of a reaction is the
chemistry-General
chemistry-
If the inversion temperature of a gas is -80°C, then it will produce cooling under Joule-Thomson effect at
If the inversion temperature of a gas is -80°C, then it will produce cooling under Joule-Thomson effect at
chemistry-General
chemistry-
For a perfectly crystalline solid  , where a and
, where a and  are constant. If
are constant. If  is 0.40 J/K mol at 10 K and 0.92 J/K mol at 20 K, then molary entropy at 20 K is :
is 0.40 J/K mol at 10 K and 0.92 J/K mol at 20 K, then molary entropy at 20 K is :
For a perfectly crystalline solid  , where a and
, where a and  are constant. If
are constant. If  is 0.40 J/K mol at 10 K and 0.92 J/K mol at 20 K, then molary entropy at 20 K is :
is 0.40 J/K mol at 10 K and 0.92 J/K mol at 20 K, then molary entropy at 20 K is :
chemistry-General
chemistry-
Two mole of an ideal gas is expanded irreversibly and isothermally at 37°C until its volume is doubled and 3.41 kJ heat is absorbed from surroundings.  (system +surroundings) is
(system +surroundings) is
Two mole of an ideal gas is expanded irreversibly and isothermally at 37°C until its volume is doubled and 3.41 kJ heat is absorbed from surroundings.  (system +surroundings) is
(system +surroundings) is
chemistry-General
chemistry-
One mole of an ideal monoatomic gas at 27°C is subjected to a reversible isoentropic compression until final temperature reached to 327°C If the initial pressure was  atm then find the value of In
atm then find the value of In 
One mole of an ideal monoatomic gas at 27°C is subjected to a reversible isoentropic compression until final temperature reached to 327°C If the initial pressure was  atm then find the value of In
atm then find the value of In 
chemistry-General
chemistry-
Calculate ΔS for 3 mole of a diatomic ideal gas which is heated and compressed from 298 K and 1 bar to 596 K and 4 bar.
Calculate ΔS for 3 mole of a diatomic ideal gas which is heated and compressed from 298 K and 1 bar to 596 K and 4 bar.
chemistry-General
chemistry-
Considering entropy(s) at a thermo dynamic parameter, the criterion for the spontanity of any process is
Considering entropy(s) at a thermo dynamic parameter, the criterion for the spontanity of any process is
chemistry-General
chemistry-
When a liquid evaporates, which is true about the signs of the enthalpy and entropy changes ?
When a liquid evaporates, which is true about the signs of the enthalpy and entropy changes ?
chemistry-General
chemistry-
When charcoal burns in air signs of ΔH and ΔS are 
When charcoal burns in air signs of ΔH and ΔS are 
chemistry-General
chemistry-
What are the signs of the entropy change (+ or - ) in the following:
I) A liquid crystallises into a solid
II) Temperature of a crystalline solid is raised from 0 K to 115 K
III) 
IV) 
What are the signs of the entropy change (+ or - ) in the following:
I) A liquid crystallises into a solid
II) Temperature of a crystalline solid is raised from 0 K to 115 K
III) 
IV) 
chemistry-General