Chemistry-
General
Easy
Question
A third group is least likely to enter between two groups in the meta relationship. This is the result of steric hindrance and increases in importance with the size of the groups on the ring and with the size of the attacking species.
When a Meta-directing group is meta to an ortho-para directing group, the incoming group primarily goes ortho to the meta directing group rather than para.





The correct answer is: 
Related Questions to study
Chemistry-
A third group is least likely to enter between two groups in the meta relationship. This is the result of steric hindrance and increases in importance with the size of the groups on the ring and with the size of the attacking species.
When a Meta-directing group is meta to an ortho-para directing group, the incoming group primarily goes ortho to the meta directing group rather than para.

A third group is least likely to enter between two groups in the meta relationship. This is the result of steric hindrance and increases in importance with the size of the groups on the ring and with the size of the attacking species.
When a Meta-directing group is meta to an ortho-para directing group, the incoming group primarily goes ortho to the meta directing group rather than para.

Chemistry-General
Chemistry-
A third group is least likely to enter between two groups in the meta relationship. This is the result of steric hindrance and increases in importance with the size of the groups on the ring and with the size of the attacking species.
When a Meta-directing group is meta to an ortho-para directing group, the incoming group primarily goes ortho to the meta directing group rather than para.

A third group is least likely to enter between two groups in the meta relationship. This is the result of steric hindrance and increases in importance with the size of the groups on the ring and with the size of the attacking species.
When a Meta-directing group is meta to an ortho-para directing group, the incoming group primarily goes ortho to the meta directing group rather than para.

Chemistry-General
Chemistry-
Benzene is converted to toluene by :
Benzene is converted to toluene by :
Chemistry-General
Chemistry-
Identify Y in the change;

Identify Y in the change;

Chemistry-General
Chemistry-
The correct order of reactivity towards electrophilic substitution is
The correct order of reactivity towards electrophilic substitution is
Chemistry-General
Chemistry-
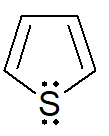
B basicity order of following heterocycles are
1) 
2) 
3) 
4) 
B basicity order of following heterocycles are
1) 
2) 
3) 
4) 
Chemistry-General
Chemistry-
Arrange the following in increasing order of electrophilic aromatic substitution.
I) 
II) 
III) 
IV) 
Arrange the following in increasing order of electrophilic aromatic substitution.
I) 
II) 
III) 
IV) 
Chemistry-General
Chemistry-
The order of the basicity in the following compounds is
I) 
II) 
III) 
IV) 
The order of the basicity in the following compounds is
I) 
II) 
III) 
IV) 
Chemistry-General
Chemistry-
Xylene on treatment with Br2/FeBr3 give only one product. Xylene was

Xylene on treatment with Br2/FeBr3 give only one product. Xylene was

Chemistry-General
Chemistry-
Which is true about the rate of nitration of the following compounds
I) 
II) 
Which is true about the rate of nitration of the following compounds
I) 
II) 
Chemistry-General
Chemistry-
Sandmeyer reaction involves the formation of
Sandmeyer reaction involves the formation of
Chemistry-General
Chemistry-
Arrange the following in increasing order of their activating capacity towards Bimolecular Aromatic substitution.
I) null,
II) CH3,
III) –NO2,
IV) null
Arrange the following in increasing order of their activating capacity towards Bimolecular Aromatic substitution.
I) null,
II) CH3,
III) –NO2,
IV) null
Chemistry-General
Chemistry-
Iodine Titration
All such titration which involves the direct titration of Iodine with a reducing agent are grouped under Iodimetry. Iodimetry is employed to determine the strength of reducing agent such as sodium those sulphate
I2 + Na2S2O3 ¾→ I– + null
If iodine is liberated as a result of chemical reaction involving oxidation of an idodide ion by a strong oxidizing agent in neutral or acidic medium the liberated iodine is then titrated with reducing agent. Iodometry is used to estimate the strength of oxidizing agent.
For example the estimation of Cu++ with thiosulphate.
Cu++ + I– ¾→Cu2I2 + I2
I2 + null
Starch used as indicator near the end point which form blue colour complex withnull. The blue colour disappears when there is no more of free I2.
100 ml of 0.1 N hypo decolourised iodine by the addition of x g of crystalline blue vitriol to excess of KI. The value of x is
Iodine Titration
All such titration which involves the direct titration of Iodine with a reducing agent are grouped under Iodimetry. Iodimetry is employed to determine the strength of reducing agent such as sodium those sulphate
I2 + Na2S2O3 ¾→ I– + null
If iodine is liberated as a result of chemical reaction involving oxidation of an idodide ion by a strong oxidizing agent in neutral or acidic medium the liberated iodine is then titrated with reducing agent. Iodometry is used to estimate the strength of oxidizing agent.
For example the estimation of Cu++ with thiosulphate.
Cu++ + I– ¾→Cu2I2 + I2
I2 + null
Starch used as indicator near the end point which form blue colour complex withnull. The blue colour disappears when there is no more of free I2.
100 ml of 0.1 N hypo decolourised iodine by the addition of x g of crystalline blue vitriol to excess of KI. The value of x is
Chemistry-General
Chemistry-
0.7 g of Na2CO3. xH2O is dissolved in 100 ml water, 20 ml of this solution required 19.8 ml of 0.1 N HCl. The value of x is
0.7 g of Na2CO3. xH2O is dissolved in 100 ml water, 20 ml of this solution required 19.8 ml of 0.1 N HCl. The value of x is
Chemistry-General
Chemistry-
0.1 g of bleaching powder, on reaction with acetic acid and excess KI solution, gave iodine which reacted with 50 ml of N/5 hypo. The per cent available Cl2 with sample is
0.1 g of bleaching powder, on reaction with acetic acid and excess KI solution, gave iodine which reacted with 50 ml of N/5 hypo. The per cent available Cl2 with sample is
Chemistry-General





