Chemistry
Grade-9
Easy
Question
How many valence electrons are elements trying to reach in their outer shells?
- 1
- 2
- 5
- 8

Noble gases have 8 electrons in their outermost shell.
The correct answer is: 8
- The electrons in the outermost shell are called valence electrons.
- Noble gases have 8 electrons in their outermost shell, the most stable electronic configuration.
- 8 valence electrons elements are trying to reach in their outer shells.
Related Questions to study
Chemistry
How would you describe reactivity on the Periodic Table?
How would you describe reactivity on the Periodic Table?
ChemistryGrade-9
Chemistry
With which element will Hydrogen most likely bond?
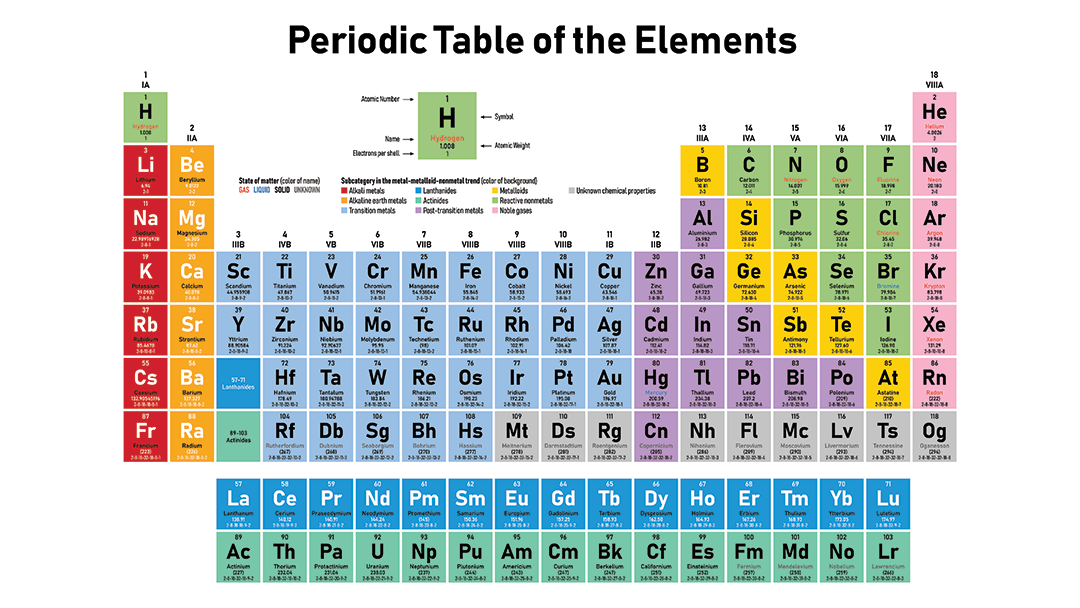
With which element will Hydrogen most likely bond?

ChemistryGrade-9
Chemistry
Maximum covalency of Nitrogen is
Maximum covalency of Nitrogen is
ChemistryGrade-9
Chemistry
Electrons that involve in bond formation are
Electrons that involve in bond formation are
ChemistryGrade-9
Chemistry
All elements in the Halogen family react violently because
All elements in the Halogen family react violently because
ChemistryGrade-9
Chemistry
Where are the transition metals found on the periodic table?
Where are the transition metals found on the periodic table?
ChemistryGrade-9
Chemistry
How many elements are in the Noble Gas family?
How many elements are in the Noble Gas family?
ChemistryGrade-9
Chemistry

Chalcogens are

Chalcogens are
ChemistryGrade-9
Chemistry
Most reactive elemental gas.
Most reactive elemental gas.
ChemistryGrade-9
Chemistry

How many valence electrons are in the element Iodine?

How many valence electrons are in the element Iodine?
ChemistryGrade-9
Chemistry
Which of the statements on physical changes are correct?
Which of the statements on physical changes are correct?
ChemistryGrade-9
Chemistry
Coinage metals are:
Coinage metals are:
ChemistryGrade-9
Chemistry
A pure substance that is a compound is made out of
A pure substance that is a compound is made out of
ChemistryGrade-9
Chemistry
Match Transition metal to its right electronic configuration.

Match Transition metal to its right electronic configuration.

ChemistryGrade-9



