Chemistry-
General
Easy
Question
Identification of cations can also be done using a dry test called sodium carbonate-bead test which is similar to borax-bead test.
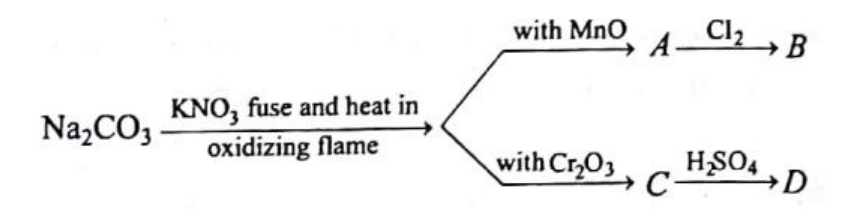
Identify A, and what is the hybridization of A?
The correct answer is: 
Related Questions to study
Chemistry-
(A) Mixture of glucose and m-dinitrobenzene can-be separated by shaking it with ether,
(R) Glucose is soluble in water.
(A) Mixture of glucose and m-dinitrobenzene can-be separated by shaking it with ether,
(R) Glucose is soluble in water.
Chemistry-General
Chemistry-
Number of and
and  bonds in C6H5COOH is:
bonds in C6H5COOH is:
Number of and
and  bonds in C6H5COOH is:
bonds in C6H5COOH is:
Chemistry-General
Chemistry-
Consider the following compounds:
A) chloroethene
B) benzene
C) buta-l,3-diene
D) 1,3,5-hexatriene
All the carbon atoms are Sp2 -hybridized in:
Consider the following compounds:
A) chloroethene
B) benzene
C) buta-l,3-diene
D) 1,3,5-hexatriene
All the carbon atoms are Sp2 -hybridized in:
Chemistry-General
Chemistry-
In the compound  the
the  bond is of the type:
bond is of the type:
In the compound  the
the  bond is of the type:
bond is of the type:
Chemistry-General
Chemistry-
Which is correct statement about azeotropic mixture?
Which is correct statement about azeotropic mixture?
Chemistry-General
Chemistry-
Anthracene is purified by:
Anthracene is purified by:
Chemistry-General
Chemistry-
Steam distillation is applied to those organic compounds which are steam volatile and:
Steam distillation is applied to those organic compounds which are steam volatile and:
Chemistry-General
Chemistry-
Latest technique for purification, isolation and separation of organic compounds is:
Latest technique for purification, isolation and separation of organic compounds is:
Chemistry-General
Chemistry-
Arrange the following compounds in order of increasing dipole moment:
i) toluene
ii) m-dichloro benzene
iii) o-dichloro benzene
iv) p-dichloro benzene
Arrange the following compounds in order of increasing dipole moment:
i) toluene
ii) m-dichloro benzene
iii) o-dichloro benzene
iv) p-dichloro benzene
Chemistry-General
Chemistry-
The maximum possible number of hydrogen bonds, a water molecule can form is:
The maximum possible number of hydrogen bonds, a water molecule can form is:
Chemistry-General
Chemistry-
Maximum amount of hydrogen bonding occurs in case of:
Maximum amount of hydrogen bonding occurs in case of:
Chemistry-General
Chemistry-
In which of the compounds below is there more than one kind of hybridization  for carbon?
for carbon?
i) 
ii) 
iii) 
iv) 
In which of the compounds below is there more than one kind of hybridization  for carbon?
for carbon?
i) 
ii) 
iii) 
iv) 
Chemistry-General
Chemistry-
Resonance in a molecule results in:
Resonance in a molecule results in:
Chemistry-General
Chemistry-
bond is the most polar.
bond is the most polar.
Chemistry-General
Chemistry-
Which of the following compounds shows evidence of the strongest hydrogen bonding?
Which of the following compounds shows evidence of the strongest hydrogen bonding?
Chemistry-General



