Chemistry-
General
Easy
Question
Two aliphatic aldehydes P and Q react in the presence of aqueous K2CO3 to give compound R, which upon treatment with HCN provides compound S On acidification and heating S gives the product shown below:
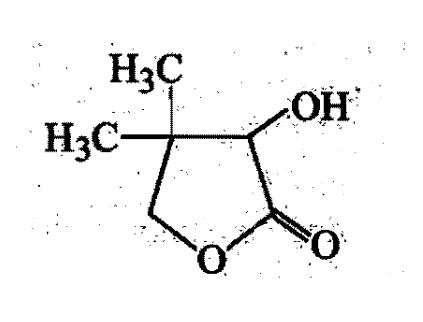
The compound P and Q respectively are :
 and
and 
 and
and 
 and
and 
 and
and 
The correct answer is:  and
and 
Related Questions to study
Chemistry-
A tertiary alcohol H on acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds J and K. Compound J upon reaction with KOH gives benzyl alcohol and a compound L, whereas K on reaction with KOH gives only M
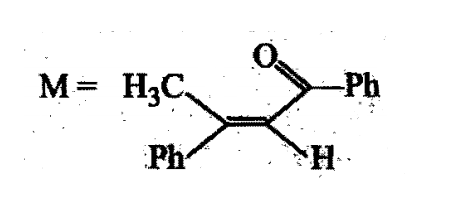
The structures of compounds J, K and L, respectively, are:
A tertiary alcohol H on acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds J and K. Compound J upon reaction with KOH gives benzyl alcohol and a compound L, whereas K on reaction with KOH gives only M

The structures of compounds J, K and L, respectively, are:
Chemistry-General
Chemistry-
A tertiary alcohol H on acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds J and K. Compound J upon reaction with KOH gives benzyl alcohol and a compound L, whereas K on reaction with KOH gives only M
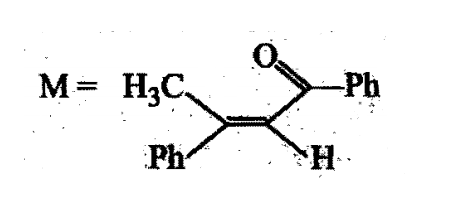
The structure of compound I is:
A tertiary alcohol H on acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds J and K. Compound J upon reaction with KOH gives benzyl alcohol and a compound L, whereas K on reaction with KOH gives only M

The structure of compound I is:
Chemistry-General
Chemistry-
A tertiary alcohol H on acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds J and K. Compound J upon reaction with KOH gives benzyl alcohol and a compound L, whereas K on reaction with KOH gives only M
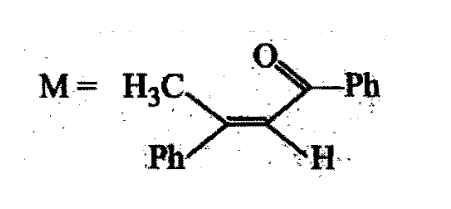
Compound H is formed by the reaction of:
A tertiary alcohol H on acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds J and K. Compound J upon reaction with KOH gives benzyl alcohol and a compound L, whereas K on reaction with KOH gives only M

Compound H is formed by the reaction of:
Chemistry-General
Chemistry-
Aldehydes undergo disproportionation reaction in presence of aqueous NaOH Simultaneous oxidation and. reduction 'of a compound is scientifically called as disproportionation.
Aldehydes having no a-hydrogen show this reaction called Cannizzaro's reaction. Few exceptions are also there to this generalization.
The reaction may be represented as:

Intramolecular Cannizzaro's reaction is also possible.
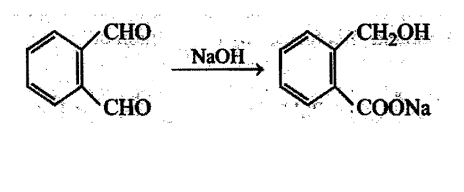
Which of the following' compounds gives internal crossed Cannizzaro's reaction?
Aldehydes undergo disproportionation reaction in presence of aqueous NaOH Simultaneous oxidation and. reduction 'of a compound is scientifically called as disproportionation.
Aldehydes having no a-hydrogen show this reaction called Cannizzaro's reaction. Few exceptions are also there to this generalization.
The reaction may be represented as:

Intramolecular Cannizzaro's reaction is also possible.

Which of the following' compounds gives internal crossed Cannizzaro's reaction?
Chemistry-General
Chemistry-
Aldehydes undergo disproportionation reaction in presence of aqueous NaOH Simultaneous oxidation and. reduction 'of a compound is scientifically called as disproportionation.
Aldehydes having no a-hydrogen show this reaction called Cannizzaro's reaction. Few exceptions are also there to this generalization.
The reaction may be represented as:

Intramolecular Cannizzaro's reaction is also possible.
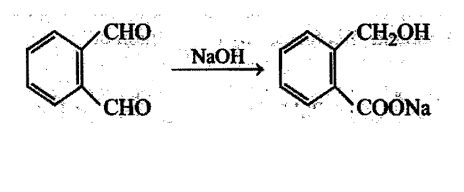
The aldehyde having  -hydrogen which gives Cannizzaro's reaction is:
-hydrogen which gives Cannizzaro's reaction is:
Aldehydes undergo disproportionation reaction in presence of aqueous NaOH Simultaneous oxidation and. reduction 'of a compound is scientifically called as disproportionation.
Aldehydes having no a-hydrogen show this reaction called Cannizzaro's reaction. Few exceptions are also there to this generalization.
The reaction may be represented as:

Intramolecular Cannizzaro's reaction is also possible.

The aldehyde having  -hydrogen which gives Cannizzaro's reaction is:
-hydrogen which gives Cannizzaro's reaction is:
Chemistry-General
Chemistry-
Aldehydes undergo disproportionation reaction in presence of aqueous NaOH Simultaneous oxidation and. reduction 'of a compound is scientifically called as disproportionation.
Aldehydes having no a-hydrogen show this reaction called Cannizzaro's reaction. Few exceptions are also there to this generalization.
The reaction may be represented as:

Intramolecular Cannizzaro's reaction is also possible.
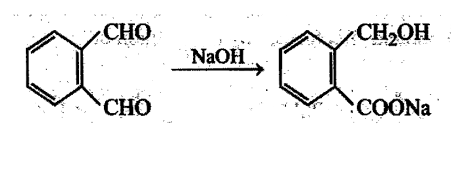
The aldehyde which shows Cannizzaro's reaction is:
Aldehydes undergo disproportionation reaction in presence of aqueous NaOH Simultaneous oxidation and. reduction 'of a compound is scientifically called as disproportionation.
Aldehydes having no a-hydrogen show this reaction called Cannizzaro's reaction. Few exceptions are also there to this generalization.
The reaction may be represented as:

Intramolecular Cannizzaro's reaction is also possible.

The aldehyde which shows Cannizzaro's reaction is:
Chemistry-General
Chemistry-
The final product (III) obtained in the reaction, 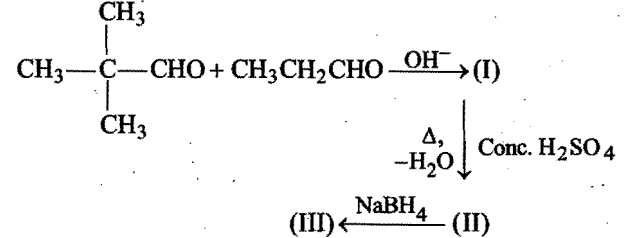 is:
is:
The final product (III) obtained in the reaction, 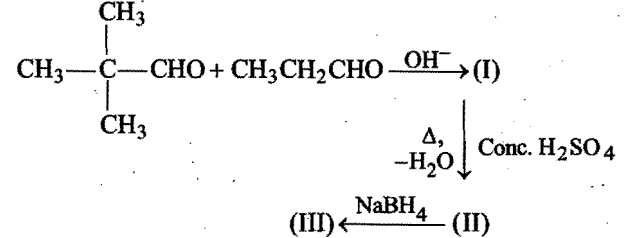 is:
is:
Chemistry-General
Chemistry-
Tishchenko reaction of 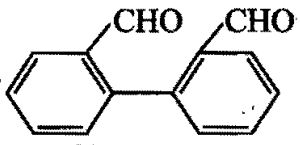 gives:
gives:
Tishchenko reaction of  gives:
gives:
Chemistry-General
Chemistry-
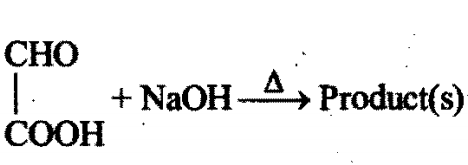 Identity the product(s):
Identity the product(s):
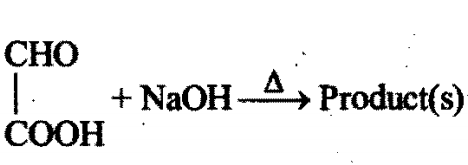 Identity the product(s):
Identity the product(s):
Chemistry-General
Chemistry-
In the reaction,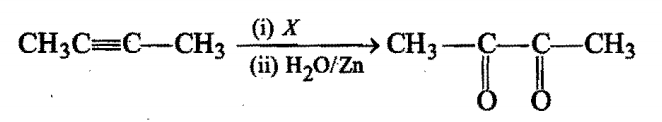 X is:
X is:
In the reaction,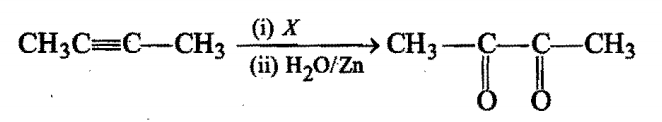 X is:
X is:
Chemistry-General
Maths-
Daniel invested in stock market. The price of one share of stock fell 12 dollars each day for 8 days. How much money did Daniel lose after 8 days
Daniel invested in stock market. The price of one share of stock fell 12 dollars each day for 8 days. How much money did Daniel lose after 8 days
Maths-General
Chemistry-
Among the following compounds, which will react. with acetone to give a product containing 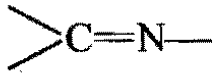 ?
?
Among the following compounds, which will react. with acetone to give a product containing  ?
?
Chemistry-General
Chemistry-
Among the given compounds, the most susceptible to nucleophilic attack at the carbonyl group is:
Among the given compounds, the most susceptible to nucleophilic attack at the carbonyl group is:
Chemistry-General
Chemistry-
In the group 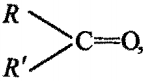 the carbonyl carbon is joined to other atoms by:
the carbonyl carbon is joined to other atoms by:
In the group  the carbonyl carbon is joined to other atoms by:
the carbonyl carbon is joined to other atoms by:
Chemistry-General
Maths-
Compare the integers.,> , < or =
-252 + 312 - 400  -212 - 452 + 300
-212 - 452 + 300
Compare the integers.,> , < or =
-252 + 312 - 400  -212 - 452 + 300
-212 - 452 + 300
Maths-General





