Chemistry
Grade-9
Easy
Question
Which one of the following is correct according to the PV vs P graph of a gas?
- Parabola
- Hyperbola
- Straight line
- Straight line parallel to x-axis

The graph of ideal gas defer from real gases.
The correct answer is: Straight line parallel to x-axis
The correct option is d)Straight line parallel to x-axis.
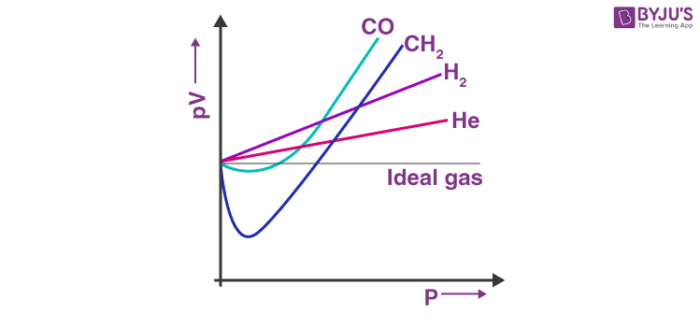
A pV-p plot is a straight line parallel to the x-axis. The figure above shows graphs generated from real data for some gases at 273 K. Looking at the graph, we can see that at constant temperature the pV vs p plot is not a straight line for real gases.
Related Questions to study
Chemistry
At constant temperature, the volume of a gas was found to be 400cm3 at a pressure of 760mm of Hg. If the pressure of the gas is increased by the half of the actual pressure find the new volume.
At constant temperature, the volume of a gas was found to be 400cm3 at a pressure of 760mm of Hg. If the pressure of the gas is increased by the half of the actual pressure find the new volume.
ChemistryGrade-9
Chemistry
Which one of the following is the real-life example of Boyle’s law?
Which one of the following is the real-life example of Boyle’s law?
ChemistryGrade-9
Chemistry
What will happen to the temperature if the pressure of a gas is increased, and its volume remains constant?
What will happen to the temperature if the pressure of a gas is increased, and its volume remains constant?
ChemistryGrade-9
Chemistry
A diver ascends to the surface of the ocean too quickly and becomes sick due to the decrease of pressure. This is an example of__________.
A diver ascends to the surface of the ocean too quickly and becomes sick due to the decrease of pressure. This is an example of__________.
ChemistryGrade-9
Chemistry
The below graph represents which gas law?

The below graph represents which gas law?

ChemistryGrade-9
Chemistry
At constant temperature the volume and the pressure associated with the gases are _____________.
At constant temperature the volume and the pressure associated with the gases are _____________.
ChemistryGrade-9
Chemistry
In Boyle’s law__________.
In Boyle’s law__________.
ChemistryGrade-9
Chemistry
At a constant volume of a gas, for a fixed number of moles, the pressure of the gas increases with an increase in temperature; this is due to:
At a constant volume of a gas, for a fixed number of moles, the pressure of the gas increases with an increase in temperature; this is due to:
ChemistryGrade-9
Chemistry
What is the pressure where one mole of a gas at 0oC occupies a volume of 1 liter?
What is the pressure where one mole of a gas at 0oC occupies a volume of 1 liter?
ChemistryGrade-9
Chemistry
Convert 6.7 L into mL
Convert 6.7 L into mL
ChemistryGrade-9
Chemistry
The best way to increase the pressure of a gas in an enclosed system
The best way to increase the pressure of a gas in an enclosed system
ChemistryGrade-9
Chemistry
Which one of the following is correct according to the STP conditions of gases?
Which one of the following is correct according to the STP conditions of gases?
ChemistryGrade-9
Chemistry
Convert 2.3 atm into mm of Hg
Convert 2.3 atm into mm of Hg
ChemistryGrade-9
Chemistry
1 liter =1000____
1 liter =1000____
ChemistryGrade-9
Chemistry
1 m3 = 1000_____.
1 m3 = 1000_____.
ChemistryGrade-9





