Physics-
General
Easy
Question
Which of the following rays are not electromagnetic waves
 -rays
-rays
 -rays
-rays
- Heat rays
- X-rays
The correct answer is:  -rays
-rays
Related Questions to study
Physics-
The ratio activity of an element becomes 1/64th of its original value in 60 sec. Then the half life period is
The ratio activity of an element becomes 1/64th of its original value in 60 sec. Then the half life period is
Physics-General
Physics-
In the disintegration series

The value of Z and A respectively will be
In the disintegration series

The value of Z and A respectively will be
Physics-General
Physics-
A radioactive nucleus with Z protons and N neutrons emits an  -particle, 2
-particle, 2  -particles and 2 gamma rays. The number of protons and neutrons in the nucleus left after the decay respectively, are
-particles and 2 gamma rays. The number of protons and neutrons in the nucleus left after the decay respectively, are
A radioactive nucleus with Z protons and N neutrons emits an  -particle, 2
-particle, 2  -particles and 2 gamma rays. The number of protons and neutrons in the nucleus left after the decay respectively, are
-particles and 2 gamma rays. The number of protons and neutrons in the nucleus left after the decay respectively, are
Physics-General
Physics-
What fraction of a radioactive material will get disintegrated in a period of two half-lives
What fraction of a radioactive material will get disintegrated in a period of two half-lives
Physics-General
Physics-
An atomic nucleus  emits several
emits several  and
and  radiations and finally reduces to
radiations and finally reduces to  . It must have emitted
. It must have emitted
An atomic nucleus  emits several
emits several  and
and  radiations and finally reduces to
radiations and finally reduces to  . It must have emitted
. It must have emitted
Physics-General
Physics-
At any instant the ratio of the amount of radioactive substances is 2 : 1. If their half lives be respectively 12 and 16 hours, then after two days, what will be the ratio of the substances
At any instant the ratio of the amount of radioactive substances is 2 : 1. If their half lives be respectively 12 and 16 hours, then after two days, what will be the ratio of the substances
Physics-General
Physics-
Radiocarbon dating is done by estimating in specimen the
Radiocarbon dating is done by estimating in specimen the
Physics-General
Physics-
If the decay constant of a radioactive substance is  , then its half-life is
, then its half-life is
If the decay constant of a radioactive substance is  , then its half-life is
, then its half-life is
Physics-General
Physics-
Which one of the following statement is true, if half-life of a radioactive substance is 1 month?
Which one of the following statement is true, if half-life of a radioactive substance is 1 month?
Physics-General
Physics-
Two nuclei have their mass numbers in the ratio of 1:3. The ratio of their nuclear densities would be
Two nuclei have their mass numbers in the ratio of 1:3. The ratio of their nuclear densities would be
Physics-General
Physics-
The above is a plot of binding energy per nucleon  against the nuclear mass
against the nuclear mass  correspond to different nuclei. Consider four reactions
correspond to different nuclei. Consider four reactions 


 where
where  is the energy released? In which reaction is
is the energy released? In which reaction is  positive?
positive?

The above is a plot of binding energy per nucleon  against the nuclear mass
against the nuclear mass  correspond to different nuclei. Consider four reactions
correspond to different nuclei. Consider four reactions 


 where
where  is the energy released? In which reaction is
is the energy released? In which reaction is  positive?
positive?

Physics-General
Chemistry-
Fe(OH)3 can be separated from Al(OH)3 by addition of
Fe(OH)3 can be separated from Al(OH)3 by addition of
Chemistry-General
Chemistry-
Which of the following elements (M) reacts with HNO3 to form MO2?
Which of the following elements (M) reacts with HNO3 to form MO2?
Chemistry-General
Chemistry-
An acidic solution contains Cu2+, Pb2+ and Zn2+. If H2S(g) is passed through the solution the precipitate will contain
An acidic solution contains Cu2+, Pb2+ and Zn2+. If H2S(g) is passed through the solution the precipitate will contain
Chemistry-General
Chemistry-
In all the oxyacids of phosphorus, each phosphorus atom is in sp3 hybrid state, i.e., it is tetrahedrally bonded to neighbouring four atoms. All these acids contain P - OH bonds, the hydrogen atom of which are ionisable imparting acidic nature to the compound. The ous acids (oxidation state of P = +1 or +3) also have P - H bonds in which hydrogens are not ionisable (P and hydrogen have nearly same electronegativity). The presence of P - H group in these acids imparts reducing properties. The structure of the various acids are drawn below (note that the tetrahedral shape of phosphorus is not shown only for convenience of representation).
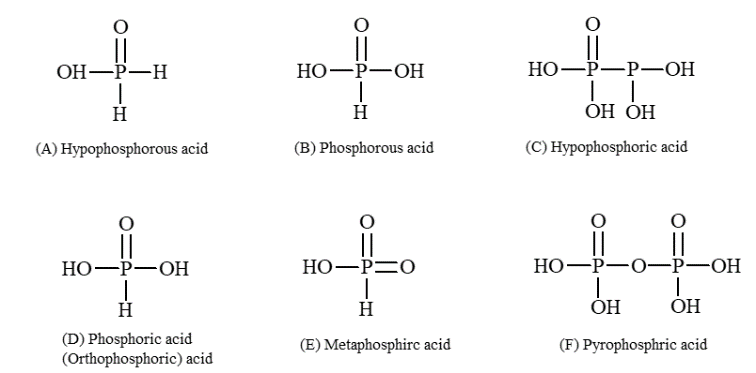
There is very little difference in acid strength in the series H3PO4, H3PO3 and H3PO2 because
In all the oxyacids of phosphorus, each phosphorus atom is in sp3 hybrid state, i.e., it is tetrahedrally bonded to neighbouring four atoms. All these acids contain P - OH bonds, the hydrogen atom of which are ionisable imparting acidic nature to the compound. The ous acids (oxidation state of P = +1 or +3) also have P - H bonds in which hydrogens are not ionisable (P and hydrogen have nearly same electronegativity). The presence of P - H group in these acids imparts reducing properties. The structure of the various acids are drawn below (note that the tetrahedral shape of phosphorus is not shown only for convenience of representation).

There is very little difference in acid strength in the series H3PO4, H3PO3 and H3PO2 because
Chemistry-General



