Chemistry-
General
Easy
Question
For hypothetical reversible 1/2A2(g)+3/2B2(g)→AB3(g);ΔH=–20KJ if stand ardentropies of A2,B2 and AB3 are60,40 and 50JK–1respectively.The above reaction will be in equilibrium at-
- 400K
- 500K
- 250K
- 200K
The correct answer is: 500K
Related Questions to study
chemistry-
For the reaction Ag2O(s)→2Ag(s)+½O2(g) the value ofΔH=30.56KJmol–1and ΔS=66 JK–1mol–1.The temperature at which the free energy change for there action will be zero is-
For the reaction Ag2O(s)→2Ag(s)+½O2(g) the value ofΔH=30.56KJmol–1and ΔS=66 JK–1mol–1.The temperature at which the free energy change for there action will be zero is-
chemistry-General
chemistry-
Which of the following is true for the r action H2O( )
) 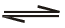 H2O(g) at 100°C and1 atmosphere-</span
H2O(g) at 100°C and1 atmosphere-</span
Which of the following is true for the r action H2O( )
) 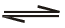 H2O(g) at 100°C and1 atmosphere-</span
H2O(g) at 100°C and1 atmosphere-</span
chemistry-General
physics
For the electrical circuit shown in the figure, the potential difference across the resistor of 400Ω as will be measured by the voltmeter of resistance 400 is…..

For the electrical circuit shown in the figure, the potential difference across the resistor of 400Ω as will be measured by the voltmeter of resistance 400 is…..

physicsGeneral
chemistry-
The temperature at which the reaction Ag2O(s)→2Ag(s)+½O2(g) is at equilibrium is.........; Given ΔH=30.5KJmol–1 and ΔS=0.066KJK–1mol–1
The temperature at which the reaction Ag2O(s)→2Ag(s)+½O2(g) is at equilibrium is.........; Given ΔH=30.5KJmol–1 and ΔS=0.066KJK–1mol–1
chemistry-General
chemistry-
If ΔH>0 and ΔS>0,thereactionproceeds spontaneously when-
If ΔH>0 and ΔS>0,thereactionproceeds spontaneously when-
chemistry-General
chemistry-
The spontaneous nature of are action is impossible if-
The spontaneous nature of are action is impossible if-
chemistry-General
chemistry-
Forareaction25°Centhalpychange(ΔH)and entropy change(ΔS)are–11.7×103Jmol–1and –105JMol–1K–1 respectively. There action is-
Forareaction25°Centhalpychange(ΔH)and entropy change(ΔS)are–11.7×103Jmol–1and –105JMol–1K–1 respectively. There action is-
chemistry-General
chemistry-
Agasis allowed to expand under reversible adiababatic conditions what is zerofor such a process-
Agasis allowed to expand under reversible adiababatic conditions what is zerofor such a process-
chemistry-General
physics
A wire is in the form of a tetrahedron shown in figure. The resistance of each wire is r. What is the resistance of he frame between the comers A and B.

A wire is in the form of a tetrahedron shown in figure. The resistance of each wire is r. What is the resistance of he frame between the comers A and B.

physicsGeneral
chemistry-
Calculate enthalpy of vapourization per mole of ethanol. Given ΔS=109.8JK–1mol–1andB.pt.of
ethanolis78.5°C.
Calculate enthalpy of vapourization per mole of ethanol. Given ΔS=109.8JK–1mol–1andB.pt.of
ethanolis78.5°C.
chemistry-General
chemistry-
The enthalpy of vaporisation of per mole of ethanol (b.p.=79.5°CandS=109.8JK–1mol–1)is-
The enthalpy of vaporisation of per mole of ethanol (b.p.=79.5°CandS=109.8JK–1mol–1)is-
chemistry-General
physics
In the above question, after appropriate conditions are made, it is found that no deflection takes places in the galvanometer where the sliding jockey touches the wire at a distance of from . What is the value of the resistance X?
In the above question, after appropriate conditions are made, it is found that no deflection takes places in the galvanometer where the sliding jockey touches the wire at a distance of from . What is the value of the resistance X?
physicsGeneral
physics
A thin uniform wire AB of length 1 m, an unknown resistance X and a resistance of  are connected, by thick conducting strips as shown in figure. A battery and a galvanometer (with a sliding jockey connected to it) are also available. Connections are to be measure the unknown resistance using the principle of Wheatstone bridge. The appropriate connections are.
are connected, by thick conducting strips as shown in figure. A battery and a galvanometer (with a sliding jockey connected to it) are also available. Connections are to be measure the unknown resistance using the principle of Wheatstone bridge. The appropriate connections are.

(E is the balance point for whetstone bridge)
A thin uniform wire AB of length 1 m, an unknown resistance X and a resistance of  are connected, by thick conducting strips as shown in figure. A battery and a galvanometer (with a sliding jockey connected to it) are also available. Connections are to be measure the unknown resistance using the principle of Wheatstone bridge. The appropriate connections are.
are connected, by thick conducting strips as shown in figure. A battery and a galvanometer (with a sliding jockey connected to it) are also available. Connections are to be measure the unknown resistance using the principle of Wheatstone bridge. The appropriate connections are.

(E is the balance point for whetstone bridge)
physicsGeneral
physics
Two liquids of equal volume are thoroughly mixed. If their specific heat are  , temperatures
, temperatures  and densities
and densities  respectively. What is the final temperature of the rrixture?
respectively. What is the final temperature of the rrixture?
Two liquids of equal volume are thoroughly mixed. If their specific heat are  , temperatures
, temperatures  and densities
and densities  respectively. What is the final temperature of the rrixture?
respectively. What is the final temperature of the rrixture?
physicsGeneral
chemistry-
For which reaction from the following,ΔSwill be maximum?
For which reaction from the following,ΔSwill be maximum?
chemistry-General



