Chemistry-
General
Easy
Question
The reactivity of acid derivatives in general follows the order :

The above order of reactivity can be explained in terms of the : (i) Basicity of leaving group (ii) Resonance effect (iii) Inductive effect Weaker is the basic character of leaving group, more is the reactivity of acid derivative. In general, all the acid derivatives show resonance as follows:

More is the stabilization, lesser is the reactivity and vice-versa.
Which of the following compounds will be most easily hydrolysed ?
- Acid halide
- Acid amide
- Ester
- Acid anhydride
The correct answer is: Acid halide
Related Questions to study
chemistry-
The reactivity of acid derivatives in general follows the order :

The above order of reactivity can be explained in terms of the : (i) Basicity of leaving group (ii) Resonance effect (iii) Inductive effect Weaker is the basic character of leaving group, more is the reactivity of acid derivative. In general, all the acid derivatives show resonance as follows:

More is the stabilization, lesser is the reactivity and vice-versa.
Acid derivatives although contain 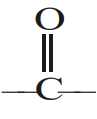 group, yet do not undergo the usual properties of carbonyl group It is due to:
group, yet do not undergo the usual properties of carbonyl group It is due to:
The reactivity of acid derivatives in general follows the order :

The above order of reactivity can be explained in terms of the : (i) Basicity of leaving group (ii) Resonance effect (iii) Inductive effect Weaker is the basic character of leaving group, more is the reactivity of acid derivative. In general, all the acid derivatives show resonance as follows:

More is the stabilization, lesser is the reactivity and vice-versa.
Acid derivatives although contain 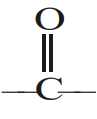 group, yet do not undergo the usual properties of carbonyl group It is due to:
group, yet do not undergo the usual properties of carbonyl group It is due to:
chemistry-General
chemistry-
The reactivity of acid derivatives in general follows the order :

The above order of reactivity can be explained in terms of the : (i) Basicity of leaving group (ii) Resonance effect (iii) Inductive effect Weaker is the basic character of leaving group, more is the reactivity of acid derivative. In general, all the acid derivatives show resonance as follows:

More is the stabilization, lesser is the reactivity and vice-versa.
Which among the following ester is most reactive towards nucleophilic attack ?
The reactivity of acid derivatives in general follows the order :

The above order of reactivity can be explained in terms of the : (i) Basicity of leaving group (ii) Resonance effect (iii) Inductive effect Weaker is the basic character of leaving group, more is the reactivity of acid derivative. In general, all the acid derivatives show resonance as follows:

More is the stabilization, lesser is the reactivity and vice-versa.
Which among the following ester is most reactive towards nucleophilic attack ?
chemistry-General
chemistry-
The reactivity of acid derivatives in general follows the order :

The above order of reactivity can be explained in terms of the : (i) Basicity of leaving group (ii) Resonance effect (iii) Inductive effect Weaker is the basic character of leaving group, more is the reactivity of acid derivative. In general, all the acid derivatives show resonance as follows:

More is the stabilization, lesser is the reactivity and vice-versa.
Which of the most reactive acid derivative?
The reactivity of acid derivatives in general follows the order :

The above order of reactivity can be explained in terms of the : (i) Basicity of leaving group (ii) Resonance effect (iii) Inductive effect Weaker is the basic character of leaving group, more is the reactivity of acid derivative. In general, all the acid derivatives show resonance as follows:

More is the stabilization, lesser is the reactivity and vice-versa.
Which of the most reactive acid derivative?
chemistry-General
chemistry-
The reactivity of acid derivatives in general follows the order :

The above order of reactivity can be explained in terms of the : (i) Basicity of leaving group (ii) Resonance effect (iii) Inductive effect Weaker is the basic character of leaving group, more is the reactivity of acid derivative. In general, all the acid derivatives show resonance as follows:

More is the stabilization, lesser is the reactivity and vice-versa.
Which among the following anions is the most basic ?
The reactivity of acid derivatives in general follows the order :

The above order of reactivity can be explained in terms of the : (i) Basicity of leaving group (ii) Resonance effect (iii) Inductive effect Weaker is the basic character of leaving group, more is the reactivity of acid derivative. In general, all the acid derivatives show resonance as follows:

More is the stabilization, lesser is the reactivity and vice-versa.
Which among the following anions is the most basic ?
chemistry-General
chemistry-
Observe the esterification mechanisms for primary and tertiary alcohols.
Type.1

Mechanism

Type. 2

Mechanism



Observe the esterification mechanisms for primary and tertiary alcohols.
Type.1

Mechanism

Type. 2

Mechanism



chemistry-General
chemistry-
Observe the esterification mechanisms for primary and tertiary alcohols.
Type.1
Mechanism

Type. 2

Mechanism



In the above reaction (P) and (Q) are respectively :
Observe the esterification mechanisms for primary and tertiary alcohols.
Type.1
Mechanism

Type. 2

Mechanism



In the above reaction (P) and (Q) are respectively :
chemistry-General
chemistry-
Observe the following sequence of reaction and answer the questions based on it

Which of the following compound give benzoic acid on KMnO4 oxidation
Observe the following sequence of reaction and answer the questions based on it

Which of the following compound give benzoic acid on KMnO4 oxidation
chemistry-General
chemistry-
Observe the following sequence of reaction and answer the questions based on it

Which of the following statement is not correct
Observe the following sequence of reaction and answer the questions based on it

Which of the following statement is not correct
chemistry-General
chemistry-
Observe the following sequence of reaction and answer the questions based on it

Compound z is :
Observe the following sequence of reaction and answer the questions based on it

Compound z is :
chemistry-General
chemistry-
In the Hofmann rearrangement an unsubstituted amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide.
General reaction :

Mechanism :

If the migrating group is chiral then its configuration is retained. Electron releasing effects in the migrating group increases reactivity of Hofmann rearrangement.

In the Hofmann rearrangement an unsubstituted amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide.
General reaction :

Mechanism :

If the migrating group is chiral then its configuration is retained. Electron releasing effects in the migrating group increases reactivity of Hofmann rearrangement.

chemistry-General
chemistry-
In the Hofmann rearrangement an unsubstituted amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide.
General reaction :

Mechanism :

If the migrating group is chiral then its configuration is retained. Electron releasing effects in the migrating group increases reactivity of Hofmann rearrangement.
Arrange the following amides according to their relative reactivity when reacted with Br2 in excess of strong base
I) 
II) 
III) 
IV) 
In the Hofmann rearrangement an unsubstituted amide is treated with sodium hydroxide and bromine to give a primary amine that has one carbon lesser than starting amide.
General reaction :

Mechanism :

If the migrating group is chiral then its configuration is retained. Electron releasing effects in the migrating group increases reactivity of Hofmann rearrangement.
Arrange the following amides according to their relative reactivity when reacted with Br2 in excess of strong base
I) 
II) 
III) 
IV) 
chemistry-General
physics-
The tube shown is of uniform cross–section. Liquid flows through it at a constant speed in the direction shown by the arrows. The liquid exerts on the tube

The tube shown is of uniform cross–section. Liquid flows through it at a constant speed in the direction shown by the arrows. The liquid exerts on the tube

physics-General
physics-
A bent arc shaped sealed glass tube filled with water is accelerated horizontally with acceleration a. A small air bubble inside it is found to be stuck at 1 cm from vertical axis. Neglect surface tension. What is the acceleration of the tube.

A bent arc shaped sealed glass tube filled with water is accelerated horizontally with acceleration a. A small air bubble inside it is found to be stuck at 1 cm from vertical axis. Neglect surface tension. What is the acceleration of the tube.

physics-General
physics-
A pan balance has a container of water with an overflow spout on the right-hand pan as shown. It is full of water right up to the overflow spout. A container on the left-hand pan is positioned to catch any water that overflows. The entire apparatus is adjusted so that it’s balanced. A brass weight on the end of a string is then lowered into the water, but not allowed to rest on the bottom of the container. What happens next?

A pan balance has a container of water with an overflow spout on the right-hand pan as shown. It is full of water right up to the overflow spout. A container on the left-hand pan is positioned to catch any water that overflows. The entire apparatus is adjusted so that it’s balanced. A brass weight on the end of a string is then lowered into the water, but not allowed to rest on the bottom of the container. What happens next?

physics-General
physics-
A cylindrical container of length L is full to the brim with a liquid which has mass density r. It is placed on a weigh-scale; the scale reading is W. A light ball which would float on the liquid if allowed to do so, of volume V and mass m is pushed gently down and held beneath the surface of the liquid with a rigid rod of negligible volume as shown on the left.

What is the reading of the scale when the ball is fully immersed?
A cylindrical container of length L is full to the brim with a liquid which has mass density r. It is placed on a weigh-scale; the scale reading is W. A light ball which would float on the liquid if allowed to do so, of volume V and mass m is pushed gently down and held beneath the surface of the liquid with a rigid rod of negligible volume as shown on the left.

What is the reading of the scale when the ball is fully immersed?
physics-General



