Maths-
General
Easy
Question
Find the number by which 7:21 is multiplied to make it equivalent to 84 : 252.
- 11
- 12
- 13
- 14
The correct answer is: 12
7:21 = 7/21 = 7x12 / 21x12 = 84 / 252
Related Questions to study
Chemistry-
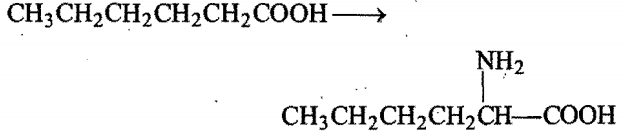 the reagents used in the conversion are:
the reagents used in the conversion are:
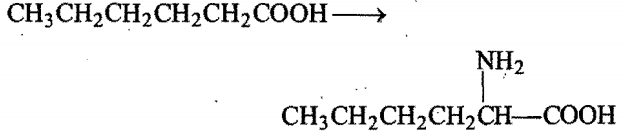 the reagents used in the conversion are:
the reagents used in the conversion are:
Chemistry-General
Chemistry-
Which is the correct order of acidity from weakest to strongest acid for these compounds:
I) CF3CH2OH
II) 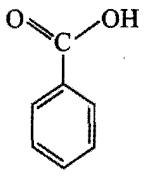
III) 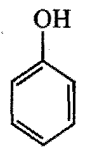
IV) 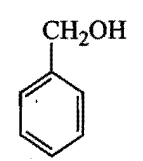
Which is the correct order of acidity from weakest to strongest acid for these compounds:
I) CF3CH2OH
II) 
III) 
IV) 
Chemistry-General
Chemistry-
Two aliphatic aldehydes P and Q react in the presence of aqueous K2CO3 to give compound R, which upon treatment with HCN provides compound S On acidification and heating S gives the product shown below:
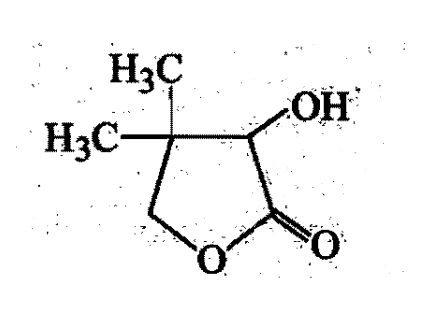
The compound P and Q respectively are :
Two aliphatic aldehydes P and Q react in the presence of aqueous K2CO3 to give compound R, which upon treatment with HCN provides compound S On acidification and heating S gives the product shown below:

The compound P and Q respectively are :
Chemistry-General
Chemistry-
A tertiary alcohol H on acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds J and K. Compound J upon reaction with KOH gives benzyl alcohol and a compound L, whereas K on reaction with KOH gives only M
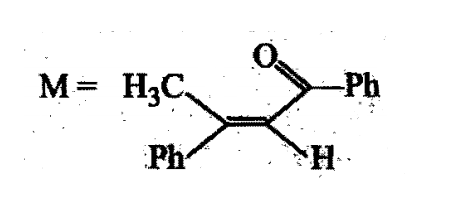
The structures of compounds J, K and L, respectively, are:
A tertiary alcohol H on acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds J and K. Compound J upon reaction with KOH gives benzyl alcohol and a compound L, whereas K on reaction with KOH gives only M

The structures of compounds J, K and L, respectively, are:
Chemistry-General
Chemistry-
A tertiary alcohol H on acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds J and K. Compound J upon reaction with KOH gives benzyl alcohol and a compound L, whereas K on reaction with KOH gives only M
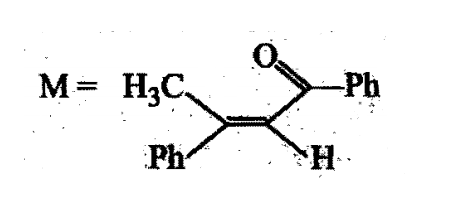
The structure of compound I is:
A tertiary alcohol H on acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds J and K. Compound J upon reaction with KOH gives benzyl alcohol and a compound L, whereas K on reaction with KOH gives only M

The structure of compound I is:
Chemistry-General
Chemistry-
A tertiary alcohol H on acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds J and K. Compound J upon reaction with KOH gives benzyl alcohol and a compound L, whereas K on reaction with KOH gives only M
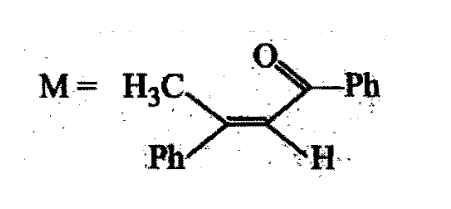
Compound H is formed by the reaction of:
A tertiary alcohol H on acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds J and K. Compound J upon reaction with KOH gives benzyl alcohol and a compound L, whereas K on reaction with KOH gives only M

Compound H is formed by the reaction of:
Chemistry-General
Chemistry-
Aldehydes undergo disproportionation reaction in presence of aqueous NaOH Simultaneous oxidation and. reduction 'of a compound is scientifically called as disproportionation.
Aldehydes having no a-hydrogen show this reaction called Cannizzaro's reaction. Few exceptions are also there to this generalization.
The reaction may be represented as:

Intramolecular Cannizzaro's reaction is also possible.
Which of the following' compounds gives internal crossed Cannizzaro's reaction?
Aldehydes undergo disproportionation reaction in presence of aqueous NaOH Simultaneous oxidation and. reduction 'of a compound is scientifically called as disproportionation.
Aldehydes having no a-hydrogen show this reaction called Cannizzaro's reaction. Few exceptions are also there to this generalization.
The reaction may be represented as:

Intramolecular Cannizzaro's reaction is also possible.

Which of the following' compounds gives internal crossed Cannizzaro's reaction?
Chemistry-General
Chemistry-
Aldehydes undergo disproportionation reaction in presence of aqueous NaOH Simultaneous oxidation and. reduction 'of a compound is scientifically called as disproportionation.
Aldehydes having no a-hydrogen show this reaction called Cannizzaro's reaction. Few exceptions are also there to this generalization.
The reaction may be represented as:

Intramolecular Cannizzaro's reaction is also possible.
The aldehyde having  -hydrogen which gives Cannizzaro's reaction is:
-hydrogen which gives Cannizzaro's reaction is:
Aldehydes undergo disproportionation reaction in presence of aqueous NaOH Simultaneous oxidation and. reduction 'of a compound is scientifically called as disproportionation.
Aldehydes having no a-hydrogen show this reaction called Cannizzaro's reaction. Few exceptions are also there to this generalization.
The reaction may be represented as:

Intramolecular Cannizzaro's reaction is also possible.

The aldehyde having  -hydrogen which gives Cannizzaro's reaction is:
-hydrogen which gives Cannizzaro's reaction is:
Chemistry-General
Chemistry-
Aldehydes undergo disproportionation reaction in presence of aqueous NaOH Simultaneous oxidation and. reduction 'of a compound is scientifically called as disproportionation.
Aldehydes having no a-hydrogen show this reaction called Cannizzaro's reaction. Few exceptions are also there to this generalization.
The reaction may be represented as:

Intramolecular Cannizzaro's reaction is also possible.
The aldehyde which shows Cannizzaro's reaction is:
Aldehydes undergo disproportionation reaction in presence of aqueous NaOH Simultaneous oxidation and. reduction 'of a compound is scientifically called as disproportionation.
Aldehydes having no a-hydrogen show this reaction called Cannizzaro's reaction. Few exceptions are also there to this generalization.
The reaction may be represented as:

Intramolecular Cannizzaro's reaction is also possible.

The aldehyde which shows Cannizzaro's reaction is:
Chemistry-General
Chemistry-
The final product (III) obtained in the reaction, 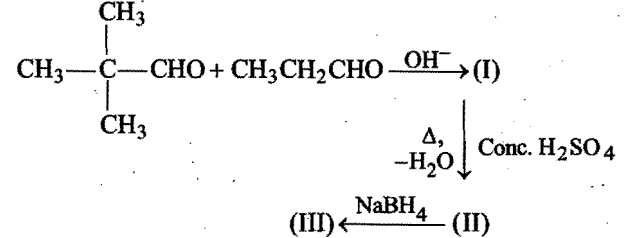 is:
is:
The final product (III) obtained in the reaction, 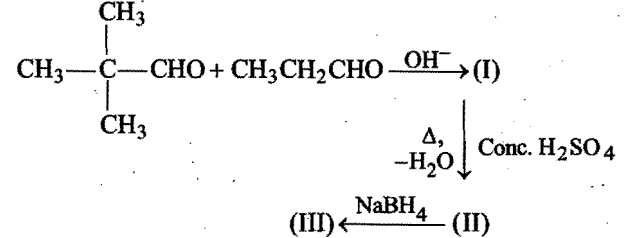 is:
is:
Chemistry-General
Chemistry-
Tishchenko reaction of 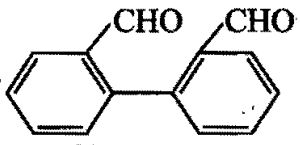 gives:
gives:
Tishchenko reaction of  gives:
gives:
Chemistry-General
Chemistry-
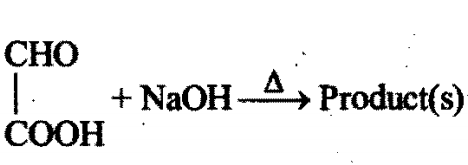 Identity the product(s):
Identity the product(s):
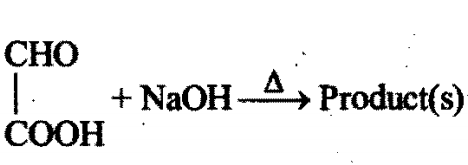 Identity the product(s):
Identity the product(s):
Chemistry-General
Chemistry-
In the reaction,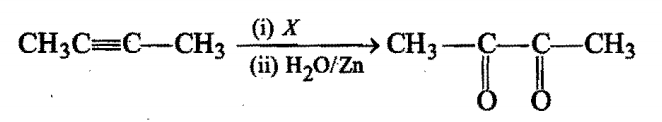 X is:
X is:
In the reaction,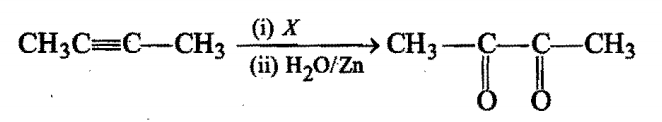 X is:
X is:
Chemistry-General
Maths-
Daniel invested in stock market. The price of one share of stock fell 12 dollars each day for 8 days. How much money did Daniel lose after 8 days
Daniel invested in stock market. The price of one share of stock fell 12 dollars each day for 8 days. How much money did Daniel lose after 8 days
Maths-General
Chemistry-
Among the following compounds, which will react. with acetone to give a product containing 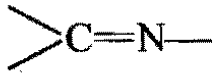 ?
?
Among the following compounds, which will react. with acetone to give a product containing  ?
?
Chemistry-General





