Maths-
General
Easy
Question
The total amount of fee collection for 21 students is $31500. How much was paid by each student.
- $1500
- $1475
- $1520
- $1375
The correct answer is: $1500
Total amount of fee collection =$31500
Total number of students =21
i.e. $31500 is distributed among 21 students.
Therefore,
Amount paid by each student be calculated as
21) 31500 (1500

Amount paid by each student is $1500
Related Questions to study
Chemistry-
 is a
is a
 is a
is a
Chemistry-General
Chemistry-
In the reaction,

In the reaction,

Chemistry-General
Chemistry-
In the given action,

the product [X] will be:
In the given action,

the product [X] will be:
Chemistry-General
Chemistry-
In the reaction sequence,

In the reaction sequence,

Chemistry-General
Chemistry-
In the given reaction,

In the given reaction,

Chemistry-General
Chemistry-
In the given reaction sequence,

the final product [BJ will be:
In the given reaction sequence,

the final product [BJ will be:
Chemistry-General
Chemistry-
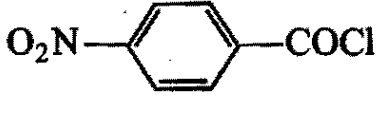
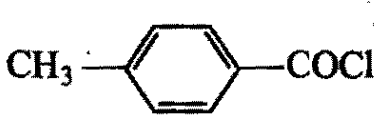
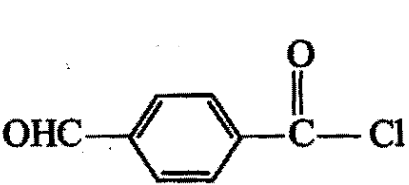
Arrange the following compounds in the decreasing order of reactivity for hydrolysis reaction:
1) 
2) 
3) 
4) 
Arrange the following compounds in the decreasing order of reactivity for hydrolysis reaction:
1) 
2) 
3) 
4) 
Chemistry-General
Chemistry-
 The product obtained in above reaction will be :
The product obtained in above reaction will be :
 The product obtained in above reaction will be :
The product obtained in above reaction will be :
Chemistry-General
Chemistry-
The percentage of nitrogen in urea is:
The percentage of nitrogen in urea is:
Chemistry-General
Maths-
In a packet of 40 pens, 12 are red. So, what % age are red pens?
In a packet of 40 pens, 12 are red. So, what % age are red pens?
Maths-General
Chemistry-
Consider the following compounds, which of these will release CO2 with 5% NaHCO3 ?
i) 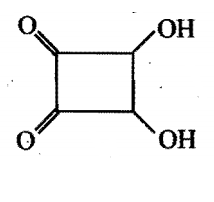
ii) 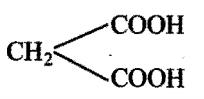
iii) 
Consider the following compounds, which of these will release CO2 with 5% NaHCO3 ?
i) 
ii) 
iii) 
Chemistry-General
Chemistry-
Identify the product' Y' in the following reaction sequence:

Identify the product' Y' in the following reaction sequence:

Chemistry-General
Chemistry-
In homogeneous catalytic reactions, there are three alternative paths A,B and C (shown in the figure) which one of the following indicates the relative ease with which the reaction can take place?

In homogeneous catalytic reactions, there are three alternative paths A,B and C (shown in the figure) which one of the following indicates the relative ease with which the reaction can take place?

Chemistry-General
Chemistry-
In a spontaneous adsorption process
In a spontaneous adsorption process
Chemistry-General
Chemistry-
Graph between log  and log P is a straight line at angle 0 45 with intercept OA as shown Hence ,
and log P is a straight line at angle 0 45 with intercept OA as shown Hence , at a pressure of 2 atm is
at a pressure of 2 atm is

Graph between log  and log P is a straight line at angle 0 45 with intercept OA as shown Hence ,
and log P is a straight line at angle 0 45 with intercept OA as shown Hence , at a pressure of 2 atm is
at a pressure of 2 atm is

Chemistry-General



