Chemistry-
General
Easy
Question
Nucleophilic substitution reaction" is given by those compounds which have nucleophilic. groups as leaving groups. The weaker the basicity of a group of the substrate, the better is its leaving ability. In nucleophilic substitution reactions, the basicity of leaving group should be less than the incoming nucleophilic group. Nucleophilic substitution reaction at sp3-hybridised carbon is either bimolecular  or 1lIlim0lecular:
or 1lIlim0lecular:  Bimolecular reaction takes place in single step, involving transition state intermediate. In
Bimolecular reaction takes place in single step, involving transition state intermediate. In  reaction, inversion in configuration takes place. In case of optically active alkyl halides, the inversion in configuration is called Walden "inversion.
reaction, inversion in configuration takes place. In case of optically active alkyl halides, the inversion in configuration is called Walden "inversion.  reaction is preferred if the compound has less steric hindrance. " Unimolecular (
reaction is preferred if the compound has less steric hindrance. " Unimolecular ( ) reaction" involves two steps and" carbonium ion intermediate. Optically active" substrates give racemic mixture in these" reactions.
) reaction" involves two steps and" carbonium ion intermediate. Optically active" substrates give racemic mixture in these" reactions.
Which among the following will give  reaction?
reaction?
I) 
II) 
III) 
IV) 
- I,II,III
- I,II,IV
- III
- II and IV
The correct answer is: I,II,IV
Related Questions to study
Chemistry-
Identify the correct order of reactivity in electrophilic substitution reactions of the following compounds:
I) 
II) 
III) 
IV) 
Identify the correct order of reactivity in electrophilic substitution reactions of the following compounds:
I) 
II) 
III) 
IV) 
Chemistry-General
Chemistry-
Dehydrobromination (HBr-) of the following in increasing order will be:
I) 
II) 
III) 
Dehydrobromination (HBr-) of the following in increasing order will be:
I) 
II) 
III) 
Chemistry-General
Chemistry-
 by the reaction is:
by the reaction is:
 by the reaction is:
by the reaction is:
Chemistry-General
Chemistry-
Oxidation of alkenes by cleavage with acidic or alkaline KMn04 or acidic K2Cr2O7 at higher temperature yields products depending upon the nature of aIkene. A hot solution ofKMnO4 is a strong oxidising agent which gives only ketones and carboxylic . acids and not aldehydes (as they cannot be isolated).
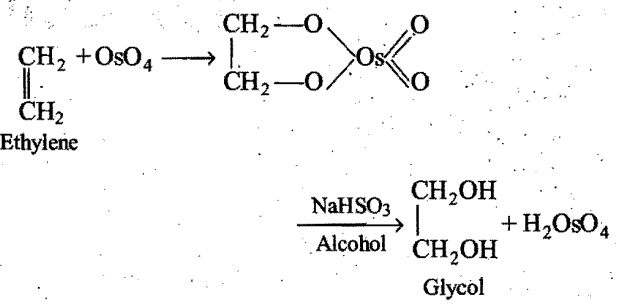
Oxidation of alkenes with OsO4 followed by alcoholic NaHSO3 or Na2SO4 yields glycols.
An alkene 1,2-dimethyl cyclobutene on oxidation With. hot KMnO4 gives:
Oxidation of alkenes by cleavage with acidic or alkaline KMn04 or acidic K2Cr2O7 at higher temperature yields products depending upon the nature of aIkene. A hot solution ofKMnO4 is a strong oxidising agent which gives only ketones and carboxylic . acids and not aldehydes (as they cannot be isolated).
Oxidation of alkenes with OsO4 followed by alcoholic NaHSO3 or Na2SO4 yields glycols.

An alkene 1,2-dimethyl cyclobutene on oxidation With. hot KMnO4 gives:
Chemistry-General
Chemistry-
Oxidation of alkenes by cleavage with acidic or alkaline KMn04 or acidic K2Cr2O7 at higher temperature yields products depending upon the nature of aIkene. A hot solution ofKMnO4 is a strong oxidising agent which gives only ketones and carboxylic . acids and not aldehydes (as they cannot be isolated).

Oxidation of alkenes with OsO4 followed by alcoholic NaHSO3 or Na2SO4 yields glycols.

An alkene 1,2-dimethyl cyclobutene on oxidation With. hot KMnO4 gives:
Oxidation of alkenes by cleavage with acidic or alkaline KMn04 or acidic K2Cr2O7 at higher temperature yields products depending upon the nature of aIkene. A hot solution ofKMnO4 is a strong oxidising agent which gives only ketones and carboxylic . acids and not aldehydes (as they cannot be isolated).

Oxidation of alkenes with OsO4 followed by alcoholic NaHSO3 or Na2SO4 yields glycols.

An alkene 1,2-dimethyl cyclobutene on oxidation With. hot KMnO4 gives:
Chemistry-General
Chemistry-
Oxidation of alkenes by cleavage with acidic or alkaline KMn04 or acidic K2Cr2O7 at higher temperature yields products depending upon the nature of aIkene. A hot solution ofKMnO4 is a strong oxidising agent which gives only ketones and carboxylic . acids and not aldehydes (as they cannot be isolated).

Oxidation of alkenes with OsO4 followed by alcoholic NaHSO3 or Na2SO4 yields glycols.

An alkene 1,2-dimethyl cyclobutene on oxidation With. hot KMnO4 gives:
Oxidation of alkenes by cleavage with acidic or alkaline KMn04 or acidic K2Cr2O7 at higher temperature yields products depending upon the nature of aIkene. A hot solution ofKMnO4 is a strong oxidising agent which gives only ketones and carboxylic . acids and not aldehydes (as they cannot be isolated).

Oxidation of alkenes with OsO4 followed by alcoholic NaHSO3 or Na2SO4 yields glycols.

An alkene 1,2-dimethyl cyclobutene on oxidation With. hot KMnO4 gives:
Chemistry-General
Maths-
Out of 21 tickets marked with numbers from 1 to 21, three are drawn at random. The chance that the numbers on them are in A.P., is
Out of 21 tickets marked with numbers from 1 to 21, three are drawn at random. The chance that the numbers on them are in A.P., is
Maths-General
Maths-
Three distinct numbers are selected from first 100 natural numbers. The probability that all the three numbers are divisible by 2 and 3 is
We should know the formula for combinations. We should know the concept of probability.
Three distinct numbers are selected from first 100 natural numbers. The probability that all the three numbers are divisible by 2 and 3 is
Maths-General
We should know the formula for combinations. We should know the concept of probability.
Maths-
Suppose values taken by a variable x are such that  , where
, where  denotes the value of x in the ith case for i = 1, 2, n. Then
denotes the value of x in the ith case for i = 1, 2, n. Then
Suppose values taken by a variable x are such that  , where
, where  denotes the value of x in the ith case for i = 1, 2, n. Then
denotes the value of x in the ith case for i = 1, 2, n. Then
Maths-General
Chemistry-
Oxidation without cleavage of sigma bond takes place in alkenes.

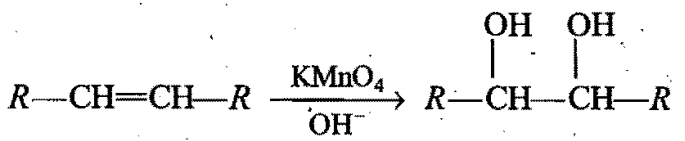
Presence of unsaturation· -in alkenes is detected by using Baeyer's reagent. Alkenes decolourise pink colour of Baeyer’s. reagent. In presence of Baeyer's reagent, 'syn' addition of-OH groups takes place on both carbons· of double· bond. The net reaction can be given as,
Ozonolysis of· alkenes give ozonide, which on further hydrolysis gives aldehyde and/or ketone.

Linear polyenes on. ozonolysis gives two moles of acetaldehyde and one mole of propanedial. Linear polyene will be:
Oxidation without cleavage of sigma bond takes place in alkenes.

Presence of unsaturation· -in alkenes is detected by using Baeyer's reagent. Alkenes decolourise pink colour of Baeyer’s. reagent. In presence of Baeyer's reagent, 'syn' addition of-OH groups takes place on both carbons· of double· bond. The net reaction can be given as,

Ozonolysis of· alkenes give ozonide, which on further hydrolysis gives aldehyde and/or ketone.

Linear polyenes on. ozonolysis gives two moles of acetaldehyde and one mole of propanedial. Linear polyene will be:
Chemistry-General
Chemistry-
Hydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the. addition ·of hydrogen takes·· place in presence of Raney nickel· catalyst. Platinum and palladium can also be used as catalyst in these reactions. These are heterogeneous catalyst and used in. finely. divided state. Experimentally, it is observed that less. crowded alkenes adsorb H2 with faster rate. Controlled hydrogeneration of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:

Non-terminal alkynes an reduce4 in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product. .
The product of the following reaction is:
<img src="https://mycourses.turito.com/tokenpluginfile.php/c161933dbfaab094c54655ab71e9b8f0/1/question/questiontext/649779/1/1178377/Picture21.png" alt="" width="285" height="89"
Hydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the. addition ·of hydrogen takes·· place in presence of Raney nickel· catalyst. Platinum and palladium can also be used as catalyst in these reactions. These are heterogeneous catalyst and used in. finely. divided state. Experimentally, it is observed that less. crowded alkenes adsorb H2 with faster rate. Controlled hydrogeneration of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:

Non-terminal alkynes an reduce4 in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product. .
The product of the following reaction is:
<img src="https://mycourses.turito.com/tokenpluginfile.php/c161933dbfaab094c54655ab71e9b8f0/1/question/questiontext/649779/1/1178377/Picture21.png" alt="" width="285" height="89"
Chemistry-General
Chemistry-
Hydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the. addition ·of hydrogen takes·· place in presence of Raney nickel· catalyst. Platinum and palladium can also be used as catalyst in these reactions. These are heterogeneous catalyst and used in. finely. divided state. Experimentally, it is observed that less. crowded alkenes adsorb H2 with faster rate. Controlled hydrogeneration of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:

Non-terminal alkynes an reduce4 in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product.
In which of the following cases, the reaction is most. exothermic?
Hydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the. addition ·of hydrogen takes·· place in presence of Raney nickel· catalyst. Platinum and palladium can also be used as catalyst in these reactions. These are heterogeneous catalyst and used in. finely. divided state. Experimentally, it is observed that less. crowded alkenes adsorb H2 with faster rate. Controlled hydrogeneration of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:

Non-terminal alkynes an reduce4 in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product.
In which of the following cases, the reaction is most. exothermic?
Chemistry-General
Chemistry-
Hydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the. addition ·of hydrogen takes·· place in presence of Raney nickel· catalyst. Platinum and palladium can also be used as catalyst in these reactions. These are heterogeneous catalyst and used in. finely. divided state. Experimentally, it is observed that less. crowded alkenes adsorb H2 with faster rate. Controlled hydrogeneration of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:

Non-terminal alkynes a reduce4 in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product.
The relative rate of catalytic hydrogenation of following. alkenes are:
I) 
II) 
III) 
IV)  </span
</span
Hydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the. addition ·of hydrogen takes·· place in presence of Raney nickel· catalyst. Platinum and palladium can also be used as catalyst in these reactions. These are heterogeneous catalyst and used in. finely. divided state. Experimentally, it is observed that less. crowded alkenes adsorb H2 with faster rate. Controlled hydrogeneration of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:

Non-terminal alkynes a reduce4 in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product.
The relative rate of catalytic hydrogenation of following. alkenes are:
I) 
II) 
III) 
IV)  </span
</span
Chemistry-General
Chemistry-
 Compounds [X] and [Y] are respectively:
Compounds [X] and [Y] are respectively:
 Compounds [X] and [Y] are respectively:
Compounds [X] and [Y] are respectively:
Chemistry-General
Chemistry-
Unknown Compound (A) on oxidation with hot basic. KMnO4 'gives only one compound whose structure is given below,

Compound (A) will be:
Unknown Compound (A) on oxidation with hot basic. KMnO4 'gives only one compound whose structure is given below,

Compound (A) will be:
Chemistry-General



