Maths-
General
Easy
Question
The focus of the parabola 
- (3, −2)
- (2, −3)
- (2, 2)
- (3, 3)
The correct answer is: (2, 2)
We know that the standard equation of a parabola is
 On comparing the given equation with the standard equation, we get the vertex as
On comparing the given equation with the standard equation, we get the vertex as
 and a = 5. Therefore, the focus of the parabola is
and a = 5. Therefore, the focus of the parabola is  = (2, 2).
= (2, 2).
Related Questions to study
Maths-
The equation of the locus of a point which moves so as to be at equal distances from the point (a, 0) and the y-axis is
The equation of the locus of a point which moves so as to be at equal distances from the point (a, 0) and the y-axis is
Maths-General
Maths-
Maths-General
Maths-
The axis of the parabola 
The axis of the parabola 
Maths-General
Maths-
Focus and directrix of the parabola 
Focus and directrix of the parabola 
Maths-General
Maths-
If the vertex of a parabola be at origin and directrix be x + = 5 0, then its latus rectum is
If the vertex of a parabola be at origin and directrix be x + = 5 0, then its latus rectum is
Maths-General
10th-Grade-Math---USA
125, 80, 140, 135, 126, 140, 350, 75
Maximum value of given data _______________
125, 80, 140, 135, 126, 140, 350, 75
Maximum value of given data _______________
10th-Grade-Math---USAGeneral
Chemistry-
 reaction is favoured by:
reaction is favoured by:
 reaction is favoured by:
reaction is favoured by:
Chemistry-General
Chemistry-
Order of base strength of the compounds :
I) 
II) 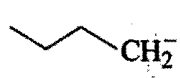
III) 
IV) 
Order of base strength of the compounds :
I) 
II) 
III) 
IV) 
Chemistry-General
Chemistry-
The stability of the following carbocation decreases in the order:
I) 
II) 
III) 
IV) 
The stability of the following carbocation decreases in the order:
I) 
II) 
III) 
IV) 
Chemistry-General
Chemistry-
Which of the following orders is correct for the ease of electrophile addition on these alkenes?
I) 
II) 
III) 
Which of the following orders is correct for the ease of electrophile addition on these alkenes?
I) 
II) 
III) 
Chemistry-General
Chemistry-
 H for CaCO3(s)
H for CaCO3(s)  CaO(s) + CO2(g) is 176 kJ mol–1 at 1240 K. The
CaO(s) + CO2(g) is 176 kJ mol–1 at 1240 K. The  E for the change is equal to
E for the change is equal to
 H for CaCO3(s)
H for CaCO3(s)  CaO(s) + CO2(g) is 176 kJ mol–1 at 1240 K. The
CaO(s) + CO2(g) is 176 kJ mol–1 at 1240 K. The  E for the change is equal to
E for the change is equal to
Chemistry-General
Chemistry-
The heat evolved in combustion if 112 litres of water gas (mixture of equal volume of H2(g) and CO(g) is
H2(g) + 1/2 O2(g) = H2O(g)  H = –241.8 kJ
H = –241.8 kJ
CO(g) + 1/2 O2(g) = CO2(g)  H = –283 kJ
H = –283 kJ
The heat evolved in combustion if 112 litres of water gas (mixture of equal volume of H2(g) and CO(g) is
H2(g) + 1/2 O2(g) = H2O(g)  H = –241.8 kJ
H = –241.8 kJ
CO(g) + 1/2 O2(g) = CO2(g)  H = –283 kJ
H = –283 kJ
Chemistry-General
Chemistry-
(1) For the given heat of reaction,
i) C(s) + O2(g) = CO2(g) + 97 kcal
ii) CO2(g) + C(s) = 2CO(g) – 39 kcal the heat of combustion of CO(g) is:
(1) For the given heat of reaction,
i) C(s) + O2(g) = CO2(g) + 97 kcal
ii) CO2(g) + C(s) = 2CO(g) – 39 kcal the heat of combustion of CO(g) is:
Chemistry-General
Chemistry-
A solution of CuSO4 is electrolysed using platinum inert electrodes. Due to electrolysis, the colour changes from blue to _________ and pH of the solution ________.
A solution of CuSO4 is electrolysed using platinum inert electrodes. Due to electrolysis, the colour changes from blue to _________ and pH of the solution ________.
Chemistry-General
Chemistry-
Chemistry-General





