Maths-
General
Easy
Question
Twelve students compete for a race. The number of ways in which first three places can be taken, is-
- 12 ! – 3

- 12
 11
11  10
10
- 3 !

There are only 3 prizes to be given to any 3 people out of 12. So if 1 person gets first prize, he will not be counted for second prize and so on....
The correct answer is: 12  11
11  10
10
The number of ways to select the winner of the first prize is 
The number of ways to select the winner of the second prize is  (11, since one student is already given a prize)
(11, since one student is already given a prize)
The number of ways to select the winner of the third prize is  (10, since two students are already given a prize)
(10, since two students are already given a prize)
Thus, total number of ways is  ×
× ×
×  = 12 × 11 × 10 = 1320
= 12 × 11 × 10 = 1320
Related Questions to study
Maths-
Given the circuit :
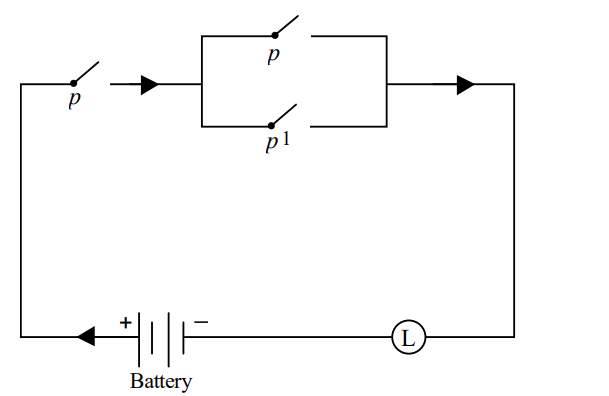
Which of the following is equivalent to the above circuit ?
I) 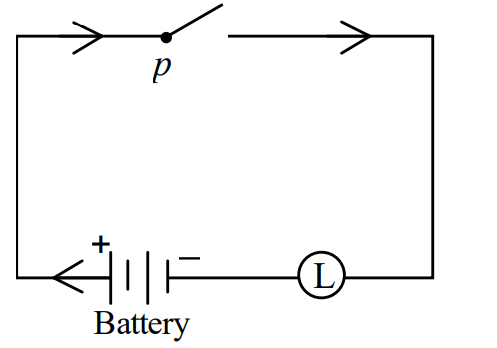
II) 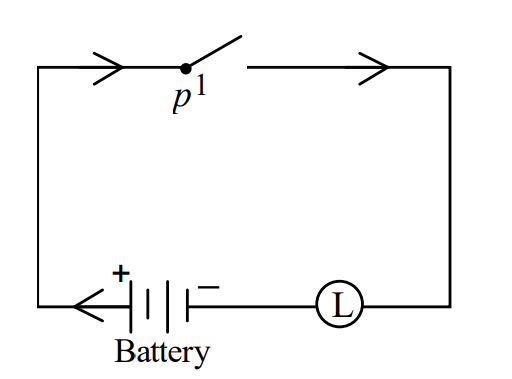
The true statement in the above is / are
Given the circuit :

Which of the following is equivalent to the above circuit ?
I) 
II) 
The true statement in the above is / are
Maths-General
Maths-
The symbolic form of logic of the circuit given below is

The symbolic form of logic of the circuit given below is

Maths-General
Maths-
The following circuit when expressed in the symbolic form of logic is

The following circuit when expressed in the symbolic form of logic is

Maths-General
chemistry-
For conversion C(graphite)→C(Diamond),the DS is-
For conversion C(graphite)→C(Diamond),the DS is-
chemistry-General
chemistry-
Consider the following processes: DH(kJ/mol) ½A→B +150 3B→2C+D–125 E+A→2D+350 ForB+D→E+2C,DHillbe-
Consider the following processes: DH(kJ/mol) ½A→B +150 3B→2C+D–125 E+A→2D+350 ForB+D→E+2C,DHillbe-
chemistry-General
chemistry-
Enthalpy change for the reaction, 4H(g) →2H2(g) is–869.6kJ The dissociation energy of H–H bond is
Enthalpy change for the reaction, 4H(g) →2H2(g) is–869.6kJ The dissociation energy of H–H bond is
chemistry-General
chemistry-
ThevaluesofDHandDSforthereaction,C(graphite)+CO(g)→2CO(g)are170kJand170 JK–1respectively.Thisreactionwillbespontaneousat
ThevaluesofDHandDSforthereaction,C(graphite)+CO(g)→2CO(g)are170kJand170 JK–1respectively.Thisreactionwillbespontaneousat
chemistry-General
maths-
Assertion: The sum of all4 digited numbers that can be formed using the digits 1,2,4,5,6 without repetition is 479952
Reason: Sum of all  ‐digit numbers formed by taking the given n digits (without zero) is (sum of all the n digits)
‐digit numbers formed by taking the given n digits (without zero) is (sum of all the n digits)  (
(  times).
times).
Assertion: The sum of all4 digited numbers that can be formed using the digits 1,2,4,5,6 without repetition is 479952
Reason: Sum of all  ‐digit numbers formed by taking the given n digits (without zero) is (sum of all the n digits)
‐digit numbers formed by taking the given n digits (without zero) is (sum of all the n digits)  (
(  times).
times).
maths-General
maths-
Assertion: There are three doors to a room The number of ways in which a student can enter the room and leave it by a different door is 6.
Reason: If an operation can be performed in  ways and another operation can be perfO rmed in
ways and another operation can be perfO rmed in  ways thenthe two operations in succession can be performed in mn ways.
ways thenthe two operations in succession can be performed in mn ways.
Assertion: There are three doors to a room The number of ways in which a student can enter the room and leave it by a different door is 6.
Reason: If an operation can be performed in  ways and another operation can be perfO rmed in
ways and another operation can be perfO rmed in  ways thenthe two operations in succession can be performed in mn ways.
ways thenthe two operations in succession can be performed in mn ways.
maths-General
maths-
Assertion(A): The number of quadratic expressions with the coefficients drawn from the set {0,1,2,3} is only 48 but not 64.
Reason(R): The coefficient  inthe quadratic expression
inthe quadratic expression  can not be
can not be  ’
’
Assertion(A): The number of quadratic expressions with the coefficients drawn from the set {0,1,2,3} is only 48 but not 64.
Reason(R): The coefficient  inthe quadratic expression
inthe quadratic expression  can not be
can not be  ’
’
maths-General
maths-
Assertion (A) : The number of ways of arranging 6 boys and 5 girls alternately at circular table is  Reason (R) : To arrange boys and girls alternately at a circular table, they should be equal in number.
Reason (R) : To arrange boys and girls alternately at a circular table, they should be equal in number.
Assertion (A) : The number of ways of arranging 6 boys and 5 girls alternately at circular table is  Reason (R) : To arrange boys and girls alternately at a circular table, they should be equal in number.
Reason (R) : To arrange boys and girls alternately at a circular table, they should be equal in number.
maths-General
chemistry-
GivenenthalpyofformationofCO2(g)andCaO(s) are–94.0KJand–152KJrespectivelyandthe enthalpyofthereaction-CaCO3(s) →CaO(s)+CO2(g) is42KJ.The enthalpyofformationofCaCO3(s)
GivenenthalpyofformationofCO2(g)andCaO(s) are–94.0KJand–152KJrespectivelyandthe enthalpyofthereaction-CaCO3(s) →CaO(s)+CO2(g) is42KJ.The enthalpyofformationofCaCO3(s)
chemistry-General
chemistry-
Change in entropy is negative for–
Change in entropy is negative for–
chemistry-General
chemistry-
The enthalpychange(DH) forthereaction,N2(g) +3H2(g) 3/4 →2NH3(g)is–92.38KJat 298K.TheinternalenergychangeDUat298Kis-
The enthalpychange(DH) forthereaction,N2(g) +3H2(g) 3/4 →2NH3(g)is–92.38KJat 298K.TheinternalenergychangeDUat298Kis-
chemistry-General
chemistry-
For a phase change H2O(l)  H2O(s)M
H2O(s)M
For a phase change H2O(l)  H2O(s)M
H2O(s)M
chemistry-General





