Chemistry-
General
Easy
Question
Fortheprocess,CO2(s)→CO2(g):
- Both ΔH and ΔS are +ve
- ΔH is negative and ΔS is +ve
- ΔH is +ve and ΔS is –ve
- Both ΔH and ΔS are –ve
The correct answer is: Both ΔH and ΔS are +ve
Related Questions to study
chemistry-
If the equilibrium constant for are action is10,then the value of ΔG°will be (R=8JK–1mol–1,T=300K)
If the equilibrium constant for are action is10,then the value of ΔG°will be (R=8JK–1mol–1,T=300K)
chemistry-General
chemistry-
What is the free energy change ΔG,when1.0mole of water at100°C and1atm pressure is converted in tosteamat100°Cand1atmpressure-
What is the free energy change ΔG,when1.0mole of water at100°C and1atm pressure is converted in tosteamat100°Cand1atmpressure-
chemistry-General
chemistry-
For the precipitation of AgCl by Ag ions and HCl
For the precipitation of AgCl by Ag ions and HCl
chemistry-General
chemistry-
For hypothetical reversible 1/2A2(g)+3/2B2(g)→AB3(g);ΔH=–20KJ if stand ardentropies of A2,B2 and AB3 are60,40 and 50JK–1respectively.The above reaction will be in equilibrium at-
For hypothetical reversible 1/2A2(g)+3/2B2(g)→AB3(g);ΔH=–20KJ if stand ardentropies of A2,B2 and AB3 are60,40 and 50JK–1respectively.The above reaction will be in equilibrium at-
chemistry-General
chemistry-
For the reaction Ag2O(s)→2Ag(s)+½O2(g) the value ofΔH=30.56KJmol–1and ΔS=66 JK–1mol–1.The temperature at which the free energy change for there action will be zero is-
For the reaction Ag2O(s)→2Ag(s)+½O2(g) the value ofΔH=30.56KJmol–1and ΔS=66 JK–1mol–1.The temperature at which the free energy change for there action will be zero is-
chemistry-General
chemistry-
Which of the following is true for the r action H2O( )
) 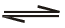 H2O(g) at 100°C and1 atmosphere-</span
H2O(g) at 100°C and1 atmosphere-</span
Which of the following is true for the r action H2O( )
) 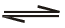 H2O(g) at 100°C and1 atmosphere-</span
H2O(g) at 100°C and1 atmosphere-</span
chemistry-General
physics
For the electrical circuit shown in the figure, the potential difference across the resistor of 400Ω as will be measured by the voltmeter of resistance 400 is…..

For the electrical circuit shown in the figure, the potential difference across the resistor of 400Ω as will be measured by the voltmeter of resistance 400 is…..

physicsGeneral
chemistry-
The temperature at which the reaction Ag2O(s)→2Ag(s)+½O2(g) is at equilibrium is.........; Given ΔH=30.5KJmol–1 and ΔS=0.066KJK–1mol–1
The temperature at which the reaction Ag2O(s)→2Ag(s)+½O2(g) is at equilibrium is.........; Given ΔH=30.5KJmol–1 and ΔS=0.066KJK–1mol–1
chemistry-General
chemistry-
If ΔH>0 and ΔS>0,thereactionproceeds spontaneously when-
If ΔH>0 and ΔS>0,thereactionproceeds spontaneously when-
chemistry-General
chemistry-
The spontaneous nature of are action is impossible if-
The spontaneous nature of are action is impossible if-
chemistry-General
chemistry-
Forareaction25°Centhalpychange(ΔH)and entropy change(ΔS)are–11.7×103Jmol–1and –105JMol–1K–1 respectively. There action is-
Forareaction25°Centhalpychange(ΔH)and entropy change(ΔS)are–11.7×103Jmol–1and –105JMol–1K–1 respectively. There action is-
chemistry-General
chemistry-
Agasis allowed to expand under reversible adiababatic conditions what is zerofor such a process-
Agasis allowed to expand under reversible adiababatic conditions what is zerofor such a process-
chemistry-General
physics
A wire is in the form of a tetrahedron shown in figure. The resistance of each wire is r. What is the resistance of he frame between the comers A and B.

A wire is in the form of a tetrahedron shown in figure. The resistance of each wire is r. What is the resistance of he frame between the comers A and B.

physicsGeneral
chemistry-
Calculate enthalpy of vapourization per mole of ethanol. Given ΔS=109.8JK–1mol–1andB.pt.of
ethanolis78.5°C.
Calculate enthalpy of vapourization per mole of ethanol. Given ΔS=109.8JK–1mol–1andB.pt.of
ethanolis78.5°C.
chemistry-General
chemistry-
The enthalpy of vaporisation of per mole of ethanol (b.p.=79.5°CandS=109.8JK–1mol–1)is-
The enthalpy of vaporisation of per mole of ethanol (b.p.=79.5°CandS=109.8JK–1mol–1)is-
chemistry-General



