Chemistry-
General
Easy
Question
Which of the follwoing reaction is expected never to be spontaneous–
- 2O3→3O2ΔH=–Ve,ΔS=+Ve
- Mg+H2→MgH2ΔH=–Ve,ΔS=–Ve
- Br2(
 )→Br2(g)ΔH=+Ve,ΔS=+Ve
)→Br2(g)ΔH=+Ve,ΔS=+Ve
- 2Ag+3N2→2AgN3ΔH=+Ve,ΔS=–Ve
The correct answer is: 2Ag+3N2→2AgN3ΔH=+Ve,ΔS=–Ve
Related Questions to study
physics
What is the resistance of an open key?
What is the resistance of an open key?
physicsGeneral
physics
a simple meter-bridge circuit, the both gaps are bridge by coils P and Q having the smaller resistance. A balance is obtained when the jockey key makes contact at a point of the bridge wire 40 cm from the P end. On shunting the coil Q with a resistance of 50Ω the balance point is moved through 10 cm . What are the resistance of P and Q
a simple meter-bridge circuit, the both gaps are bridge by coils P and Q having the smaller resistance. A balance is obtained when the jockey key makes contact at a point of the bridge wire 40 cm from the P end. On shunting the coil Q with a resistance of 50Ω the balance point is moved through 10 cm . What are the resistance of P and Q
physicsGeneral
chemistry-
The purification of alumina is called
The purification of alumina is called
chemistry-General
chemistry-
TheGibbsfreeenergychangeofareactionat27°C is –26 Kcal and its entropy change –60Cals/K.ΔH for the reaction is-
TheGibbsfreeenergychangeofareactionat27°C is –26 Kcal and its entropy change –60Cals/K.ΔH for the reaction is-
chemistry-General
chemistry-
Fortheprocess,CO2(s)→CO2(g):
Fortheprocess,CO2(s)→CO2(g):
chemistry-General
chemistry-
If the equilibrium constant for are action is10,then the value of ΔG°will be (R=8JK–1mol–1,T=300K)
If the equilibrium constant for are action is10,then the value of ΔG°will be (R=8JK–1mol–1,T=300K)
chemistry-General
chemistry-
What is the free energy change ΔG,when1.0mole of water at100°C and1atm pressure is converted in tosteamat100°Cand1atmpressure-
What is the free energy change ΔG,when1.0mole of water at100°C and1atm pressure is converted in tosteamat100°Cand1atmpressure-
chemistry-General
chemistry-
For the precipitation of AgCl by Ag ions and HCl
For the precipitation of AgCl by Ag ions and HCl
chemistry-General
chemistry-
For hypothetical reversible 1/2A2(g)+3/2B2(g)→AB3(g);ΔH=–20KJ if stand ardentropies of A2,B2 and AB3 are60,40 and 50JK–1respectively.The above reaction will be in equilibrium at-
For hypothetical reversible 1/2A2(g)+3/2B2(g)→AB3(g);ΔH=–20KJ if stand ardentropies of A2,B2 and AB3 are60,40 and 50JK–1respectively.The above reaction will be in equilibrium at-
chemistry-General
chemistry-
For the reaction Ag2O(s)→2Ag(s)+½O2(g) the value ofΔH=30.56KJmol–1and ΔS=66 JK–1mol–1.The temperature at which the free energy change for there action will be zero is-
For the reaction Ag2O(s)→2Ag(s)+½O2(g) the value ofΔH=30.56KJmol–1and ΔS=66 JK–1mol–1.The temperature at which the free energy change for there action will be zero is-
chemistry-General
chemistry-
Which of the following is true for the r action H2O( )
) 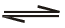 H2O(g) at 100°C and1 atmosphere-</span
H2O(g) at 100°C and1 atmosphere-</span
Which of the following is true for the r action H2O( )
) 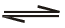 H2O(g) at 100°C and1 atmosphere-</span
H2O(g) at 100°C and1 atmosphere-</span
chemistry-General
physics
For the electrical circuit shown in the figure, the potential difference across the resistor of 400Ω as will be measured by the voltmeter of resistance 400 is…..

For the electrical circuit shown in the figure, the potential difference across the resistor of 400Ω as will be measured by the voltmeter of resistance 400 is…..

physicsGeneral
chemistry-
The temperature at which the reaction Ag2O(s)→2Ag(s)+½O2(g) is at equilibrium is.........; Given ΔH=30.5KJmol–1 and ΔS=0.066KJK–1mol–1
The temperature at which the reaction Ag2O(s)→2Ag(s)+½O2(g) is at equilibrium is.........; Given ΔH=30.5KJmol–1 and ΔS=0.066KJK–1mol–1
chemistry-General
chemistry-
If ΔH>0 and ΔS>0,thereactionproceeds spontaneously when-
If ΔH>0 and ΔS>0,thereactionproceeds spontaneously when-
chemistry-General
chemistry-
The spontaneous nature of are action is impossible if-
The spontaneous nature of are action is impossible if-
chemistry-General



