Physics-
General
Easy
Question
A pulse shown here is reflected from the rigid wall A and then from free end B. The shape of the string after these 2 reflection will be




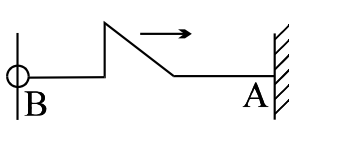
The correct answer is: 
Related Questions to study
physics-
A block of mass 1 kg is hanging vertically from a string of length 1 m and mass/length = 0.001 Kg/m. A small pulse is generated at its lower end. The pulse reaches the top end in approximately

A block of mass 1 kg is hanging vertically from a string of length 1 m and mass/length = 0.001 Kg/m. A small pulse is generated at its lower end. The pulse reaches the top end in approximately

physics-General
physics-
Figure shown the shape of part of a long string in which transverse waves are produced by attaching one end of the string to tuning fork of frequency 250 Hz. What is the velocity of the waves?

Figure shown the shape of part of a long string in which transverse waves are produced by attaching one end of the string to tuning fork of frequency 250 Hz. What is the velocity of the waves?

physics-General
physics-
The equation of a wave travelling along the positive x-axis, as shown in figure at t = 0 is given by

The equation of a wave travelling along the positive x-axis, as shown in figure at t = 0 is given by

physics-General
physics-
An enclosed ideal gas is taken through a cycle as shown in the figure. Then

An enclosed ideal gas is taken through a cycle as shown in the figure. Then

physics-General
physics-
Two moles of monoatomic gas is expanded from (P0 , V0 ) to (P0 , 2V0 ) under isobaric condition. Let DQ1 , be the heat given to the gas, DW1 the work done by the gas and DU1 the change in internal energy. Now the monoatomic gas is replaced by a diatomic gas. Other conditions remaining the same. The corresponding values in this case are  ,
,  ,
,  respectively, then
respectively, then
Two moles of monoatomic gas is expanded from (P0 , V0 ) to (P0 , 2V0 ) under isobaric condition. Let DQ1 , be the heat given to the gas, DW1 the work done by the gas and DU1 the change in internal energy. Now the monoatomic gas is replaced by a diatomic gas. Other conditions remaining the same. The corresponding values in this case are  ,
,  ,
,  respectively, then
respectively, then
physics-General
physics-
A student records  for a thermodynamic cycle
for a thermodynamic cycle  . Certain entries are missing. Find correct entry in following options.
. Certain entries are missing. Find correct entry in following options.

A student records  for a thermodynamic cycle
for a thermodynamic cycle  . Certain entries are missing. Find correct entry in following options.
. Certain entries are missing. Find correct entry in following options.

physics-General
physics-
Figure shows the pressure P versus volume V graphs for two different gas sample at a given temperature. MA and MB are masses of two samples, nA and nB are numbers of moles. Which of the following must be incorrect.

Figure shows the pressure P versus volume V graphs for two different gas sample at a given temperature. MA and MB are masses of two samples, nA and nB are numbers of moles. Which of the following must be incorrect.

physics-General
physics-
An ideal gas expands in such a way that PV2 = constant throughout the process.
An ideal gas expands in such a way that PV2 = constant throughout the process.
physics-General
physics-
A process is shown in the diagram. Which of the following curves may represent the same process ?

A process is shown in the diagram. Which of the following curves may represent the same process ?

physics-General
physics-
A resistance coil connected to an external battery is placed inside an adiabatic cylinder fitted with a frictionless piston and containing an ideal gas. A current i flows through the coil which has a resistance R. At what speed must the piston move upward in order that the temperature of the gas remains unchanged? Neglect atmospheric pressure.

A resistance coil connected to an external battery is placed inside an adiabatic cylinder fitted with a frictionless piston and containing an ideal gas. A current i flows through the coil which has a resistance R. At what speed must the piston move upward in order that the temperature of the gas remains unchanged? Neglect atmospheric pressure.

physics-General
physics-
Considere the thermodynamics cycle shown on PV diagram. The process  is isobaric,
is isobaric,  is isochoric and C →A is a straight line process. The following internal energy and heat are given
is isochoric and C →A is a straight line process. The following internal energy and heat are given  = – 500 kJ The heat flow in the process
= – 500 kJ The heat flow in the process  is :
is :

Considere the thermodynamics cycle shown on PV diagram. The process  is isobaric,
is isobaric,  is isochoric and C →A is a straight line process. The following internal energy and heat are given
is isochoric and C →A is a straight line process. The following internal energy and heat are given  = – 500 kJ The heat flow in the process
= – 500 kJ The heat flow in the process  is :
is :

physics-General
physics-
A cyclic process ABCA is shown in PT diagram. When presented on PV, it would

A cyclic process ABCA is shown in PT diagram. When presented on PV, it would

physics-General
physics-
An ideal gas undergoes a thermodynamics cycle as shown in figure. Which of the following graphs represents the same cycle?

An ideal gas undergoes a thermodynamics cycle as shown in figure. Which of the following graphs represents the same cycle?

physics-General
physics-
A closed container is fully insulated from outside. One half of it is filled with an ideal gas X separated by a plate P from the other half Y which contains a vacuum as shown in figure. When P is removed, X moves into Y. Which of the following statements is correct?

A closed container is fully insulated from outside. One half of it is filled with an ideal gas X separated by a plate P from the other half Y which contains a vacuum as shown in figure. When P is removed, X moves into Y. Which of the following statements is correct?

physics-General
physics-
Three processes compose a thermodynamics cycle shown in the PV diagram. Process 1→2 takes place at constant temperature. Process 2→3 takes place at constant volume, and process 3→1 is adiabatic. During the complete cycle, the total amount of work done is 10 J. During process 2→3, the internal energy decrease by 20J and during process 3→1, 20 J of work is done on the system. How much heat is added to the system during process 1→2?

Three processes compose a thermodynamics cycle shown in the PV diagram. Process 1→2 takes place at constant temperature. Process 2→3 takes place at constant volume, and process 3→1 is adiabatic. During the complete cycle, the total amount of work done is 10 J. During process 2→3, the internal energy decrease by 20J and during process 3→1, 20 J of work is done on the system. How much heat is added to the system during process 1→2?

physics-General





