Question
Oxidation reaction involves loss of electrons, and reduction reaction involves gain of electrons. The reaction in which a species disproportionate into two oxidation states (lower and higher) is called disproportionate reaction Which of the following statements is wrong?
- An acidified
 paper on being exposed to
paper on being exposed to  turns green
turns green
- Mercuric chloride and stannous chloride cannot exist as such
- Iron turning on addition to
 solution Decolourises the blue Colour
solution Decolourises the blue Colour  is formed but
is formed but  is not
is not
The correct answer is:  is formed but
is formed but  is not
is not
Rotation of area about incident light doesn’t change the inclination of the light ray on the area.
Related Questions to study
This section contains five paragraphs. Based on each paragraph, 3-7 multiple choice questions have to be answered. Each question has four choices (a) , (b) , (c) , and (d) , out of which only one is correct, except in the paragraph for problem 19-25 Consider the following unbalanced redox reaction:
The oxidation number of X is-2, and neither X nor water is involved in the redox process
The element(s) undergoing oxidation is/are
This section contains five paragraphs. Based on each paragraph, 3-7 multiple choice questions have to be answered. Each question has four choices (a) , (b) , (c) , and (d) , out of which only one is correct, except in the paragraph for problem 19-25 Consider the following unbalanced redox reaction:
The oxidation number of X is-2, and neither X nor water is involved in the redox process
The element(s) undergoing oxidation is/are
Statement 1: and
are both bleaching agents
Statement 2:Both are reducing agents
Statement 1: and
are both bleaching agents
Statement 2:Both are reducing agents
Statement 1:Reduction of 3-phenyl prop-2-en-1-al with LAH gives 3-phenyl prpan-1-ol
Statement 2:Both the double bond and the aldehyde group of unsaturated aldehydes are reduced by LAH
Statement 1:Reduction of 3-phenyl prop-2-en-1-al with LAH gives 3-phenyl prpan-1-ol
Statement 2:Both the double bond and the aldehyde group of unsaturated aldehydes are reduced by LAH
Statement 1:A reaction between Fe and I2 occurs, but a reaction between and
does not occur
Statement 2:Fe is a better reducing agent than
Statement 1:A reaction between Fe and I2 occurs, but a reaction between and
does not occur
Statement 2:Fe is a better reducing agent than
If  then
then 
If  then
then 
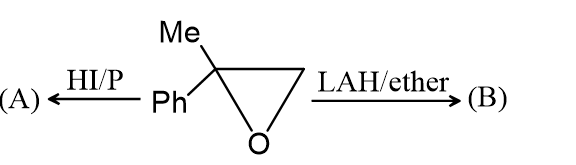 The Products (A) and (B) , respectively, are:
The Products (A) and (B) , respectively, are:
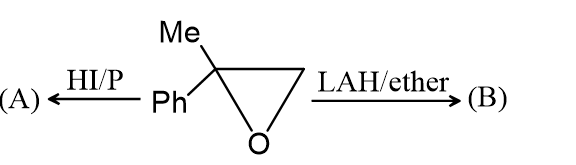 The Products (A) and (B) , respectively, are:
The Products (A) and (B) , respectively, are:
The cartesian equation of the plane passing through the line of intersection of the planes  and
and  and perpendicular to the plane
and perpendicular to the plane  is
is
The cartesian equation of the plane passing through the line of intersection of the planes  and
and  and perpendicular to the plane
and perpendicular to the plane  is
is
Identify(A) and (B) in the reaction:

Identify(A) and (B) in the reaction:

If  then at
then at  is
is
If  then at
then at  is
is
In the reaction 
In the reaction 
 The product (A) formed can:
The product (A) formed can:
 The product (A) formed can:
The product (A) formed can:
Which of the following is the strongest oxidising agent?
Which of the following is the strongest oxidising agent?
Two circles with radii  , touch each other externally. If
, touch each other externally. If  be the angle between the direct common tangents, then
be the angle between the direct common tangents, then
Two circles with radii  , touch each other externally. If
, touch each other externally. If  be the angle between the direct common tangents, then
be the angle between the direct common tangents, then
In triangle ABC, medians AD and BE are drawn. If AD=4, and
, then the area of the △ABC is
In triangle ABC, medians AD and BE are drawn. If AD=4, and
, then the area of the △ABC is



