Chemistry-
General
Easy
Question
Acetic acid is a weak electrolyte because:
- its molecular mass is high
- it in covalent compound
- ltls11ignly unstable
- it does not dissociate much, or its ionisation is very small
The correct answer is: it does not dissociate much, or its ionisation is very small
Related Questions to study
Physics-
A body is projected horizontally from the top of a high tower with a speed of 20 m/s. After 4 sec the displacement of the body is (take g=10m/s2)
A body is projected horizontally from the top of a high tower with a speed of 20 m/s. After 4 sec the displacement of the body is (take g=10m/s2)
Physics-General
Chemistry-
Consider the reaction: The rate equation for this reaction is :
The rate equation for this reaction is :  Which of these mechanisms is/are consistent with this rate equation ?
Which of these mechanisms is/are consistent with this rate equation ?
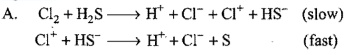

Consider the reaction: The rate equation for this reaction is :
The rate equation for this reaction is :  Which of these mechanisms is/are consistent with this rate equation ?
Which of these mechanisms is/are consistent with this rate equation ?
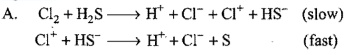

Chemistry-General
Physics-
Two particles move in a uniform gravitational field with an acceleration g. At the initial moment the particles were located at one point and moved with velocities V1 = 1 m/s and V2 = 4 m/s horizontally in opposite directions. The distance between the particles at the moment when their velocity vectors become mutually perpendicular is (g=10m/s2)
Two particles move in a uniform gravitational field with an acceleration g. At the initial moment the particles were located at one point and moved with velocities V1 = 1 m/s and V2 = 4 m/s horizontally in opposite directions. The distance between the particles at the moment when their velocity vectors become mutually perpendicular is (g=10m/s2)
Physics-General
Chemistry-
An endothermic reaction with high activation energy for the forward reaction is given by the diagram:
An endothermic reaction with high activation energy for the forward reaction is given by the diagram:
Chemistry-General
Chemistry-
Graph between concentration of the product' x' and time' t' for  is given ahead:
is given ahead:
 The graph between
The graph between  and time wil1 be of the type:
and time wil1 be of the type:
Graph between concentration of the product' x' and time' t' for  is given ahead:
is given ahead:
 The graph between
The graph between  and time wil1 be of the type:
and time wil1 be of the type:
Chemistry-General
Chemistry-
Following is the graph between  and time t for second order reaction.
and time t for second order reaction.  hence rate at the start of reaction will be:
hence rate at the start of reaction will be:

Following is the graph between  and time t for second order reaction.
and time t for second order reaction.  hence rate at the start of reaction will be:
hence rate at the start of reaction will be:

Chemistry-General
Chemistry-
Following is the graph between log tll2 and log a (a = initial concentration) for a given
 reaction at
reaction at  e. Hence, order is:
e. Hence, order is:
Following is the graph between log tll2 and log a (a = initial concentration) for a given
 reaction at
reaction at  e. Hence, order is:
e. Hence, order is:
Chemistry-General
Chemistry-
For the reaction  the probable mechanism is,
the probable mechanism is,
 The rate law will be:
The rate law will be:
For the reaction  the probable mechanism is,
the probable mechanism is,
 The rate law will be:
The rate law will be:
Chemistry-General
Chemistry-
A drop of solution (volume 0.05 mL) contains  mole of W. If the rate constant of disappearance of H+ is 107 mol
mole of W. If the rate constant of disappearance of H+ is 107 mol , how long would it take for W in the drop tell disappear?
, how long would it take for W in the drop tell disappear?
A drop of solution (volume 0.05 mL) contains  mole of W. If the rate constant of disappearance of H+ is 107 mol
mole of W. If the rate constant of disappearance of H+ is 107 mol , how long would it take for W in the drop tell disappear?
, how long would it take for W in the drop tell disappear?
Chemistry-General
Chemistry-
P4 + 5O2 ¾ → X  Y, Y is
Y, Y is
P4 + 5O2 ¾ → X  Y, Y is
Y, Y is
Chemistry-General
Chemistry-
Cu2+ + 2e– ¾® Cu; log[Cu2+] vs Ered graph is of the type shown in figure where OA = 0.34V, then electrode potential of the half cell of Cu/Cu2+ (0.1 M) will be

Cu2+ + 2e– ¾® Cu; log[Cu2+] vs Ered graph is of the type shown in figure where OA = 0.34V, then electrode potential of the half cell of Cu/Cu2+ (0.1 M) will be

Chemistry-General
Chemistry-
Which statement is correct for alkaline earth metals
Which statement is correct for alkaline earth metals
Chemistry-General
Chemistry-
The incorrect statement is
The incorrect statement is
Chemistry-General
Chemistry-
Ketone can be prepared by
Ketone can be prepared by
Chemistry-General
Chemistry-
Chemistry-General





