Question
Redox equations are balanced either by ion-electron method or by oxidation number method. Both methods lead to the correct from of the balanced equation. The ion electron method has two advantages. So some chemists prefer to use the ion-electron method for redox reactions carried out in dilute aqueous solutions, where free ions have more or less independent existence The oxidation state method for redox reactions is mostly used for solid chemicals or for reactions in concentrated acid media For the reaction The -factor is
- 1
- 11

- 61
The correct answer is: 61
A narrow pulse is made of harmonic waves with a large range of wavelength. As speed of propagation is different for different wavelengths, the pulse cannot retain its shape while travelling through the medium.
Related Questions to study
The valency of carbon is generally 4, but its oxidation state may be -4,-2,0,+2,-1 etc. In the compounds containing C,H, and O, the oxidation number of C is calculated as Oxidation number of Where
and nc are the number of oxygen, hydrogen and carbon atoms , respectively The oxidation state of C in diamond is
The valency of carbon is generally 4, but its oxidation state may be -4,-2,0,+2,-1 etc. In the compounds containing C,H, and O, the oxidation number of C is calculated as Oxidation number of Where
and nc are the number of oxygen, hydrogen and carbon atoms , respectively The oxidation state of C in diamond is
Oxidation reaction involves loss of electrons, and reduction reaction involves gain of electrons. The reaction in which a species disproportionate into two oxidation states (lower and higher) is called disproportionate reaction Which of the following statements is wrong?
Oxidation reaction involves loss of electrons, and reduction reaction involves gain of electrons. The reaction in which a species disproportionate into two oxidation states (lower and higher) is called disproportionate reaction Which of the following statements is wrong?
This section contains five paragraphs. Based on each paragraph, 3-7 multiple choice questions have to be answered. Each question has four choices (a) , (b) , (c) , and (d) , out of which only one is correct, except in the paragraph for problem 19-25 Consider the following unbalanced redox reaction:
The oxidation number of X is-2, and neither X nor water is involved in the redox process
The element(s) undergoing oxidation is/are
This section contains five paragraphs. Based on each paragraph, 3-7 multiple choice questions have to be answered. Each question has four choices (a) , (b) , (c) , and (d) , out of which only one is correct, except in the paragraph for problem 19-25 Consider the following unbalanced redox reaction:
The oxidation number of X is-2, and neither X nor water is involved in the redox process
The element(s) undergoing oxidation is/are
Statement 1: and
are both bleaching agents
Statement 2:Both are reducing agents
Statement 1: and
are both bleaching agents
Statement 2:Both are reducing agents
Statement 1:Reduction of 3-phenyl prop-2-en-1-al with LAH gives 3-phenyl prpan-1-ol
Statement 2:Both the double bond and the aldehyde group of unsaturated aldehydes are reduced by LAH
Statement 1:Reduction of 3-phenyl prop-2-en-1-al with LAH gives 3-phenyl prpan-1-ol
Statement 2:Both the double bond and the aldehyde group of unsaturated aldehydes are reduced by LAH
Statement 1:A reaction between Fe and I2 occurs, but a reaction between and
does not occur
Statement 2:Fe is a better reducing agent than
Statement 1:A reaction between Fe and I2 occurs, but a reaction between and
does not occur
Statement 2:Fe is a better reducing agent than
If  then
then 
If  then
then 
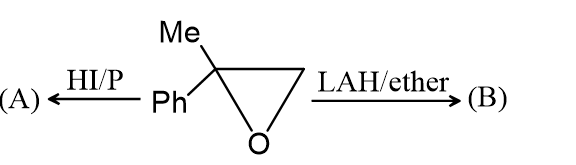 The Products (A) and (B) , respectively, are:
The Products (A) and (B) , respectively, are:
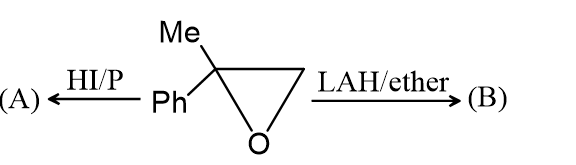 The Products (A) and (B) , respectively, are:
The Products (A) and (B) , respectively, are:
The cartesian equation of the plane passing through the line of intersection of the planes  and
and  and perpendicular to the plane
and perpendicular to the plane  is
is
The cartesian equation of the plane passing through the line of intersection of the planes  and
and  and perpendicular to the plane
and perpendicular to the plane  is
is
Identify(A) and (B) in the reaction:

Identify(A) and (B) in the reaction:

If  then at
then at  is
is
If  then at
then at  is
is
In the reaction 
In the reaction 
 The product (A) formed can:
The product (A) formed can:
 The product (A) formed can:
The product (A) formed can:
Which of the following is the strongest oxidising agent?
Which of the following is the strongest oxidising agent?



